
What is hydroxyapatite and why do we need it?
Like fluoride, a mineral that keeps teeth strong and resistant to acids formed by bacteria in dental plaque. But have you heard of hydroxyapatite? This is a mineral that occurs naturally in teeth and bones.
What are the disadvantages of hydroxyapatite?
The drawback being that calcium citrate supplements contain very little calcium, which means very little gets to your bones to improve bone strength and health. The Bottom Line In combination with vitamin D3, hydroxyapatite can effectively protect your bones from age-related degeneration and reduce the risk of fracture and disease.
What is calcium hydroxyapatite made of?
Calcium hydroxyapatite contains the amino acids, phosphate, and other essential minerals that human bones need. Calcium hydroxyapatite’s similarity to the composition of natural bone allows for optimal bioavailability and stability over other calcium phosphate ingredients.
What is hydroxyapatite toothpaste and how does it work?
Different types of toothpaste have special ingredients that help prevent and reduce dental health issues. Like fluoride, a mineral that keeps teeth strong and resistant to acids formed by bacteria in dental plaque. But have you heard of hydroxyapatite? This is a mineral that occurs naturally in teeth and bones.
What is the function of hydroxyapatite in bone quizlet?
The hydroxyapatite crystals give bones their hardness and strength, while the collagen fibers give them flexibility so that they are not brittle. synthesize and secrete the collagen matrix and calcium salts.
Why is hydroxyapatite important in bone?
Hydroxyapatite (HA) is an essential element required for bone regeneration. Different forms of HA have been used for a long time. The essence of bone regeneration always revolves around the healthy underlying bone or it may be the surroundings that give enough strength.
How does hydroxyapatite affect bone?
It possesses unique properties, such as the ability to chemically bond to bone, lack of toxicity or inflammation, and ability to stimulate bone growth through a direct action on osteoblasts. HA is widely used in periodontology and in oral and maxillofacial surgery.
Where is hydroxyapatite found in bone?
Hydroxyapatite is present in bone and teeth; bone is made primarily of HA crystals interspersed in a collagen matrix—65 to 70% of the mass of bone is HA. Similarly HA is 70 to 80% of the mass of dentin and enamel in teeth.
What minerals do hydroxyapatite provide store for the body?
Bone composition and structure Sixty-five percent of bone tissue is inorganic mineral, which provides the hardness of bone. The major minerals found in bone are calcium and phosphorus in the form of an insoluble salt called hydroxyapatite [chemical formula: (Ca)10(PO4)6(OH)2].
What are the 2 components of hydroxyapatite?
Hydroxyapatite (HA) is an inorganic mineral that has a typical apatite lattice structure as (A10(BO4)6C2) where A, B, and C are defined by Ca, PO4, and OH. Pure HA contains 39.68% by weight calcium and 18% by weight phosphorus resulting in a Ca/P mole ratio of 1.67.
Does hydroxyapatite increase bone density?
In a small placebo-controlled randomised trial, women who took 1000 mg of calcium in the form of hydroxyapatite in conjunction with oral Vit D showed a significant increase in bone thickness, whereas those who took 1000 mg of a standard calcium carbonate supplement did not (figure 4).
What is hydroxyapatite made from?
Hydroxyapatite is a naturally occurring form of the mineral calcium apatite—calcium, phosphorous, and oxygen—that grows in hexagonal crystals.
Are bones made of hydroxyapatite?
Bone is a composite tissue consisting of mineral, matrix (collagen and non-collagenous proteins), cells, and water. The mineral is hydroxyapatite, which is an analog of the naturally occurring crystalline calcium phosphate.
Which toothpaste has the most hydroxyapatite?
Best hydroxyapatite toothpastesTwice Oral Wellness Toothpaste Charcoal Icy Mint. Twice.Tend Tender Toothpaste. Tend.Boka Lemon Lavender Natural Toothpaste. ... Davids Sensitive + Whitening Premium Toothpaste. ... vVardis White Enamel Anti-Aging Toothpaste. ... Bite Fresh Mint Toothpaste Bits. ... RiseWell Natural Hydroxyapatite Toothpaste.
What foods contain calcium hydroxyapatite?
Bone Broth Protein.Egg White Protein.Pea Protein.Plant Protein.Rice Protein.Soy Protein.Whey & Casein Protein.
What is the source of calcium hydroxyapatite?
animal bonesCalcium Hydroxyapatite and Microcrystalline Hydroxyapatite Complex: Although many foods provide various calcium salts, human and animal bones are the only natural source of calcium hydroxyapatite.
Does hydroxyapatite increase bone density?
In a small placebo-controlled randomised trial, women who took 1000 mg of calcium in the form of hydroxyapatite in conjunction with oral Vit D showed a significant increase in bone thickness, whereas those who took 1000 mg of a standard calcium carbonate supplement did not (figure 4).
Are bones made of hydroxyapatite?
Bone is a composite tissue consisting of mineral, matrix (collagen and non-collagenous proteins), cells, and water. The mineral is hydroxyapatite, which is an analog of the naturally occurring crystalline calcium phosphate.
How is hydroxyapatite formed in bone?
Bone mineral The deposition of calcium and phosphate into hydroxyapatite occurs initially by nucleation of extracellular, lipid bilayer-enclosed microstructures (matrix vesicles) released by cells but ultimately requires collagen fibrils.
Why hydroxyapatite is used in implant fabrication?
Hydroxyapatite is the main inorganic component of bones and teeth. It has been extensively used as an implant material for bone substitute owing to its excellent biocompatible properties. Hydroxyapatite based composite materials shows significant properties in the field of biomedical applications.
How are hydroxyapatite ceramics made?
Dense hydroxyapatite ceramics are made by compressing calcium phosphate solids from a very fine powder form , known as greenstate, into a pellet which is then subjected to a heat treatment that causes the powder particles to fuse together by means of solid-state diffusion.
Can calcium hydroxyapatite cause arthritis?
Deposition of microcrystals such as ... calcium hydroxyapatite into joints can cause acute or chronic arthritis in a manner similar to that caused by monosodium urate deposition.
What is hydroxyapatite used for?
Hydroxyapatite (HT), (Ca5 (PO 4) 3 OH) 2, is a form of calcium phosphate used in the chromatographic separation of biomolecules. Five calcium doublets (Ca sites) and pairs of -OH containing phosphate triplets (P sites) are arranged in a repeating geometric pattern. Hydroxyapatite has unique separation properties, such as high selectivity and resolution, and can be used in separations of proteins by electrophoretic and chromatographic techniques. Applications of hydroxyapatite chromatography include the purification of different subclasses of monoclonal and polyclonal antibodies, antibody subtypes with different chain compositions, antibody fragments, isozymes, supercoiled DNA from linear duplexes, and differentiating single-strand from double-strand DNA [60].
What is the chemical formula for hydroxyapatite?
Hydroxyapatite (HA) is a calcium phosphate mineral with the chemical formula Ca10 (PO 4) 6 (OH) 2. HA-like compounds compose approximately 65% of bone, making it an appealing option for a synthetic bone composite [55].
What is HA in dentistry?
Hydroxyapatite (HA or HAP) of biologic (coral-, bovine- or marine algae-derived) or synthetic origin are currently used for bone repair and bone regeneration in the form of granules, blocks and scaffolds, by itself or as composite with polymers or other ceramics or as coatings on orthopedic or dental implants.
What are HAp materials?
HAp materials have also garnered attention in soft tissue regeneration due to their excellent biocompatibility with soft tissues such as skin, muscle, and gums. The studies showed that the HAp can activate fibroblasts and accumulate vessel endothelial cells and thereby support the healing of skin wounds ( Okabayashi et al., 2009 ). HAp bioceramics contact tightly and adhere strongly with skin tissue to prevent exit-site and tunnel bacterial infection, which suggests that HAp materials might be utilized as percutaneous devices ( Shin et al., 1992 ). The composite products with a HAp component have been successfully developed for soft-tissue augmentation ( Ji et al., 2012 ), and a study has demonstrated that HAp nanoparticles could stimulate the axonal out-growth, suggesting that HAp might provide a new approach for therapy or prevention of nerve injury ( Liu et al., 2012a ).
What is HA polyethylene?
HA–polyethylene composites with HA contents between 20 and 40 vol% show ductility and Young's moduli similar to bone, as well as bioactivity, i.e. bone-bonding ability. The optimum composition is 40 vol% of HA particles because the fracture toughness of the composite remains significantly high with high bioactivity. The combination of HA and polyethylene gives a novel bone-repairing material matching the mechanical properties of bone. This composite material is used as a trimmable orbital implant for orbital floor fractures and for volume augmentation, and as trimmable shafts for middle ear implants.
Why is HA used as a bone substitute?
HA is also used as abrasives to roughen metal implant surfaces and as source material for depositing bioactive coatings on orthopedic and dental implants.
Which type of CHT has the best binding capacity?
CHT Type I has a higher protein-binding capacity and better capacity for acidic proteins, while CHT Type II has a lower overall protein-binding capacity but has better resolution for nucleic acids and certain proteins.
Where is hydroxyapatite found?
Hydroxyapatite is present in bone and teeth; bone is made primarily of HA crystals interspersed in a collagen matrix—65 to 70% of the mass of bone is HA. Similarly HA is 70 to 80% of the mass of dentin and enamel in teeth. In enamel, the matrix for HA is formed by amelogenins and enamelins instead of collagen.
How is hydroxyapatite synthesized?
Hydroxyapatite can be synthesized via several methods, such as wet chemical deposition, biomimetic deposition, sol-gel route (wet-chemical precipitation) or electrodeposition. The hydroxyapatite nanocrystal suspension can be prepared by a wet chemical precipitation reaction following the reaction equation below:
What is the hydroxyl endmember of a complex apatite group?
Hydroxyapatite is the hydroxyl endmember of the complex apatite group. The OH − ion can be replaced by fluoride, chloride or carbonate, producing fluorapatite or chlorapatite. It crystallizes in the hexagonal crystal system. Pure hydroxyapatite powder is white.
What is the formula for hydroxyapatite?
Hydroxyapatite, also called hydroxylapatite ( HA ), is a naturally occurring mineral form of calcium apatite with the formula Ca 5 (PO 4) 3 (OH), but it is usually written Ca 10 (PO 4) 6 (OH) 2 to denote that the crystal unit cell comprises two entities. Hydroxyapatite is the hydroxyl endmember of the complex apatite group.
What is the name of the mixture of tricalcium phosphate and hydroxyapatite?
Sintering these non-stoichiometric phases forms a solid phase which is an intimate mixture of tricalcium phosphate and hydroxyapatite, termed biphasic calcium phosphate:
Does nano hydroxyapatite help with dentine hypersensitivity?
In comparison to alternative treatments for dentine hypersensitivity relief nano-hydroxyapatite containing treatment has been shown to perform better clinically. Nano-hydroxyapatite was proven to be better than other treatments at reducing sensitivity against evaporative stimuli, such as an air blast, and tactile stimuli, such as tapping the tooth with a dental instrument. However, no difference was seen between nano-hydroxyapatite and other treatments for cold stimuli. Hydroxyapatite has shown significant medium and long-term desensitizing effects on dentine hypersensitivity using evaporative stimuli and the visual analogue scale (alongside potassium nitrate, arginine, glutaraldehyde with hydroxyethyl methacrylate, hydroxyapatite, adhesive systems, glass ionomer cements and laser).
Can you synthetically replicate hydroxyapatite?
The ability to synthetically replicate hydroxyapatite has invaluable clinical implications, especially in dentistry. Each technique yields hydroxyapatite crystals of varied characteristics, such as size and shape. These variations have a marked effect on the biological and mechanical properties of the compound, and therefore these hydroxyapatite products have different clinical uses.
What is hydroxyapatite?
Hydroxyapatite (HA) is a ceramic material which forms the mineral phase of bone. It is comprised primarily of calcium and phosphate at a respective ratio of 1.67. HA has been used extensively within the world of orthopedics as a biomaterial to promote tissue regeneration. Nanoscale formulations of HA have been investigated for both orthopedic and dental tissue regenerative applications. When in its nanoscale formulation, this material has proven advantageous due to its rapid rate of resorption, which has been to shown to result from its high solubility due to its nanocrystallinity [ 38 ].
What are nanoparticles used for?
Inorganic nanoparticles have been incorporated into tissue-engineered scaffolds in order to improve the mechanical properties. As previously discussed, nHAp commonly functions to improve mechanical strength and bioactivity within a tissue-engineered scaffold, but additional nanoscale ceramics, specifically bioactive glasses, which are bone repair materials comprised of SiO 2, CaO, P 2 O 5, and Na 2 O at different ratios, have begun to gain popularity for similar reasons. The incorporation of nanoscale bioglass with a molar percent composition of SiO 2 :CaO:P 2 O 5 (55:40:5) into chitosan membranes for periodontal tissue regeneration increased stiffness and osteoconductivity of the scaffold [ 47 ]. Additionally, exposing cementoblasts to nanoscale bioactive glass (79% SiO 2, 19% CaO, 2% P 2 O 5) led to increased viability, metabolic activity, and proliferation [ 48 ]. Similarly, Gaharwar et al. have shown that the incorporation of nanosilicate into poly (ethylene glycol) (PEG) hydrogels can increase the mechanical strength of the scaffolds. Furthermore, MC3T3-E1 mouse pre-osteoblast cells were shown to adhere and spread on the scaffolds. Therefore, these scaffolds may be appropriate candidates for use in orthopedic tissue regeneration [ 49 ].
What is nhap coated titanium?
These implants have been coated with nHAp in an attempt to make them bioactive. nHAp coated on titanium implants can lead to improved mechanical strength at the tissue-im plant interface [ 39 ]. When sand blasted, acid etched titanium implants coated with hydroxyapatite were placed in rabbits, bones closest to the implants displayed improved elastic modulus and hardness when evaluated with nanoindentation. This result was attributed to calcium and phosphate release from the coating, resulting in enhanced mineralization. Furthermore, the authors reported that the nanoscale formulation itself may have enhanced mineralization. Additionally, enhanced ALP expression, which may have resulted from the nHAp coating, could have increased OPN expression which, as explained previously, is a known cohesive factor for mineralization. Coated titanium implants hold promise for craniofacial tissue engineering as well. In fact, incorporation of nHAp not only renders a titanium implant bone bonding, but it has been found that the underlying surface roughness leads to a complex surface structure, which further improves bonding at the tissue and implant interface [ 40 ].
How is rapid prototyping used in bone engineering?
Furthermore, that approach can enable the development of dimensionally accurate prototypes of bone , which is an attractive feature for bone engineering. Solid free-form fabrication allows the production of inorganic complex-shaped scaffolds directly from 3D objects fabricated using computer-aided design [86]. For that, an HAp slurry is produced by mixing HAp powder with a freezing vehicle (e.g., camphene). Then the slurry is placed into a mold previously produced using a stereolithography device and allowed to cool. Finally, it is freeze-dried to remove the freezing vehicle and sintered to remove the mold and any freezing vehicle still present, which densifies the scaffold. This method allows the production of pores 100 μm in diameter. A similar approach that creates custom scaffolds is 3D printing [87]. The scaffold can be produced with complex internal structures and high resolution. Scaffolds prepared by this method had smaller pores (10–60 μm) and a porosity of about 50%. In addition, the compressive strength (0.88 MPa) was close to the lower limit of cancellous bone.
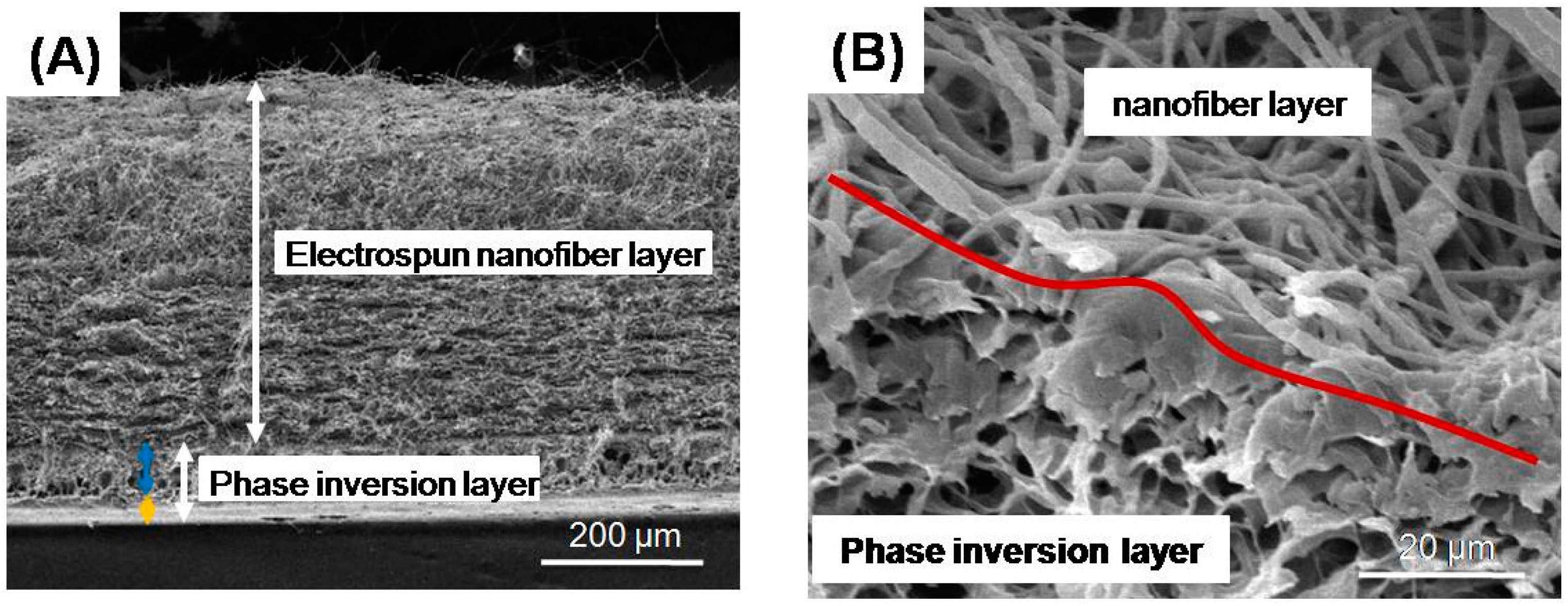
How did Franco and al. produce fibers of HAp of submicron dimensions?
Franco et al. produced fibers of HAp of submicron dimensions using electrospinning [89]. They took advantage of the gel phase produced during the sol–gel technique for HAp production. At the end, the fibers were sintered. This technique produced fibers 122 nm in diameter for the lower viscosity tested and 567 nm for the higher viscosity.
How is HAp made?
Synthetic HAp can be produced by different methods [1]: hydrothermal (in the presence of water and at a high temperature and pressure) [2]; sol–gel (formed by apatite crystals at a low temperature and pressure owing to the high reactivity of calcium and phosphorus reagents used) [3]; wet chemical (by mixing an aqueous precursor solution or hydrolyzing calcium phosphate); and [4] biomimetic deposition (the nucleation of HAp when subjected to body fluids) [84]. In addition, parameters such as the pH, reaction time, temperature, and concentration of reagents during synthesis can influence the obtained HAp.
What is HA in dentistry?
Hydroxyapatite (HA) is a ceramic material which forms the mineral phase of bone. It is comprised primarily of calcium and phosphate at a respective ratio of 1.67. HA has been used extensively within the world of orthopedics as a biomaterial to promote tissue regeneration. Nanoscale formulations of HA have been investigated for both orthopedic and dental tissue regenerative applications. When in its nanoscale formulation, this material has proven advantageous due to its rapid rate of resorption, which has been to shown to result from its high solubility due to its nanocrystallinity [ 38 ].
What Is Hydroxyapatite?
Hydroxyapatite (HA) is one of the building blocks that make up your tooth enamel, dentin, and cementum. This naturally occurring mineral gives your teeth and bones their hard and strong quality. But did you know that plaque, as well as foods high in sugar and acidic drinks, can negatively affect your teeth? You can maintain your oral health and reduce the risk of enamel erosion by using toothpaste or mouthrinse that contains hydroxyapatite.
How Does Hydroxyapatite Help Teeth Overall?
Hydroxyapatite can help protect your tooth's outer layer, prevent demineralization, and rebuild tooth enamel. This can also reduce:
Does toothpaste contain hydroxyapatite?
Some toothpaste and mouthrinses contain a nanocrystal form of hydroxyapatite (nHA) or a zinc combination (Zn-HA). According to an article published in Materials, hydroxyapatite toothpaste can counteract the effects of sodas, energy drinks, and other acidic beverages.
Can you use hydroxyapatite toothpaste on tooth sensitivity?
This can also reduce: The next time you're at the dentist, remember to ask about hydroxyapatite toothpaste. If you experience enamel mineral loss or tooth sensitivity, your dentist may recommend a remineralizing toothpaste. This will help repair the outer layer of your teeth and improve your dental health too.
What hormone is needed for calcium absorption?
The hormone calcitriol is required for calcium absorption, and vitamin D3 contributes to the formation of this hormone. Without calcium absorption, the body must get the calcium it needs from additional sources, your bones. Calcium that is stored in the bones is removed and used as needed, which leads to weakened bones and the prevention ...
Why do you need calcium carbonate?
Calcium carbonate needs to be taken with meals because it requires a large amount of stomach acid to digest. This means that very little calcium is actually absorbed, less calcium reaches your bones, and more supplements are needed . Calcium citrate is another common calcium supplement that is more easily absorbed than calcium carbonate.
Is calcium hydroxyapatite good for bone health?
Calcium Hydroxyapatite and How to Best Protect Your Bones. Some have referred to calcium hydroxyapatite as the future of bone health. It delivers a more bioavailable and more efficient form of calcium than other supplements. Here's what you need to know about adding it to your bone health regimen.
Is hydroxyapatite absorbed by the body?
Calcium hydroxyapatite is more readily absorbed as compared to other calcium supplements, but regulation of calcium in the body depends on more than just calcium. Vitamin D3 plays an integral role in maintaining calcium balance in the body.
