What is pyruvate dehydrogenase (PDH)?
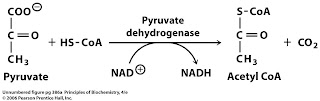
The pyruvate dehydrogenase (PDH) enzyme is part of the multienzyme PDC, which catalyzes the physiologically irreversible decarboxylation of pyruvate to acetyl-CoA and is often referred to as a ‘gatekeeper’ in the oxidation of carbohydrate (Figure 3 ).
How do you regulate pyruvate dehydrogenase activity?
Regulation of Pyruvate Dehydrogenase Activity. The pyruvate dehydrogenase complex catalyzes an irreversible reaction that is the entry point of pyruvate into the TCA cycle (see following text) and is under complex regulation by allosteric and covalent modification of the pyruvate dehydrogenase component of the complex.
What is the function of E2 in pyruvate dehydrogenase?
Function. Lipoic acid is covalently bound to dihydrolipoamide acetyltransferase (E2), which is the second catalytic component enzyme of PDC. The reaction catalyzed by pyruvate dehydrogenase (E1) is considered to be the rate-limiting step for the pyruvate dehydrogenase complex (PDHc).
What is the normal function of pyruvate dehydrogenase?
Pyruvate dehydrogenase (PDH) is a convergence point in the regulation of the metabolic finetuning between glucose and FA oxidation. Hence, PDH converts pyruvate to acetyl-coA, and thereby increases the influx of acetyl-coA from glycolysis into the TCA cycle.
Where does pyruvate dehydrogenase work?
In eukaryotes, the pyruvate dehydrogenase complex, like the enzymes for citric acid cycle and oxidation of fatty acids, is located in the mitochondrion, where is associated with the surface of the inner membrane facing the matrix. In prokaryotes, it is located in the cytosol.
What is pyruvate dehydrogenase activity?
Pyruvate dehydrogenase is a mitochondrial multienzyme complex that catalyzes the conversion of pyruvate to acetyl-coenzyme A and regulates the entry of carbohydrate into the tricarboxylic acid cycle for oxidation.
What is the function of pyruvate dehydrogenase class 11?
Pyruvate dehydrogenase is the enzyme that catalyzes the oxidation of pyruvate, by decarboxylating it to release carbon dioxide and it adds a coenzyme molecule to synthesize acetyl-CoA.
What does a dehydrogenase enzyme do?
Dehydrogenases are enzymes that catalyze reduction reactions through the transfer of hydrogen ions (protons) from the substrate to an acceptor or co-enzyme.
Why is it called pyruvate dehydrogenase?
Acetyl-CoA may then be used in the citric acid cycle to carry out cellular respiration, and this complex links the glycolysis metabolic pathway to the citric acid cycle. Pyruvate decarboxylation is also known as the "pyruvate dehydrogenase reaction" because it also involves the oxidation of pyruvate.
What class of enzyme is pyruvate dehydrogenase?
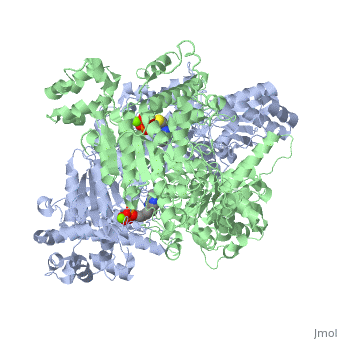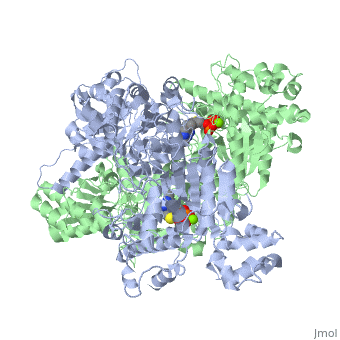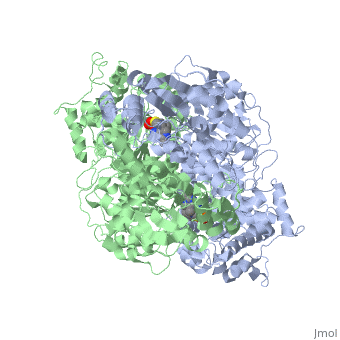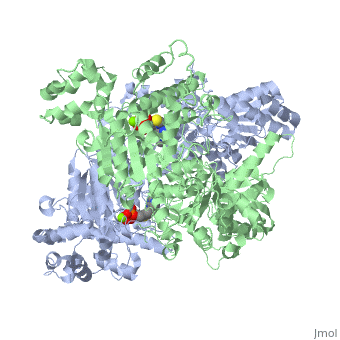
alpha and beta proteinsPyruvate dehydrogenase (E1) falls within the class of alpha and beta proteins, containing mixed alpha helices and beta sheets. It is a multimeric protein. Mammalian E1s, including human E1, are heterotetrameric, composed of two α- and two β- subunits.
Is pyruvate dehydrogenase part of glycolysis?
Under aerobic conditions, the pyruvate produced by glycolysis will be oxidized to acetyl-CoA using the pyruvate dehydrogenase complex (PDC). This enzyme is a key transition point between cytosolic and mitochondrial metabolism.
Where is pyruvate dehydrogenase most likely active?
PDC is located in the mitochondrial matrix space, and is responsible for irreversibly converting pyruvate into acetyl CoA, the primary fuel of the citric acid cycle (CAC). Reactions of the CAC and fatty acid oxidation are performed in the mitochondrial matrix.
Is pyruvate dehydrogenase in the mitochondria?
Pyruvate dehydrogenase and carboxylase enzymes are localized to the mitochondrial matrix and, therefore, pyruvate must be transported from the cytosol through both the outer and the inner mitochondrial membranes.
Where is pyruvate dehydrogenase found quizlet?
Where is pyruvate dehydrogenase found in the cell? In the mitochondria matrix.
Is pyruvate dehydrogenase part of glycolysis?
Under aerobic conditions, the pyruvate produced by glycolysis will be oxidized to acetyl-CoA using the pyruvate dehydrogenase complex (PDC). This enzyme is a key transition point between cytosolic and mitochondrial metabolism.
What is the binding protein of pyruvate dehydrogenase?
in eukaryotes, a binding protein called E3BP. The pyruvate dehydrogenase complex catalyzes, through five sequential reactions, the oxidative decarboxylation of pyruvate, an α-keto acid, to form a carbon dioxide molecules (CO 2) and the acetyl group of acetyl-coenzyme A or acetyl-CoA, with the release of two electrons, carried by NAD.
What are the coenzymes used in pyruvate dehydrogenase?
Five coenzymes are used in the pyruvate dehydrogenase complex reactions: thiamine pyrophosphate or TPP, flavin adenine dinucleotide or FAD, coenzyme A or CoA, nicotinamide adenine dinucleotide or NAD, and lipoic acid.
What is the PDC complex?
The pyruvate dehydrogenase complex (PDC) is a mitochondrial multienzyme complex composed of three different enzymes:
How many pyruvate molecules are in a glucose molecule?
Under physiological conditions, in most cells it derives mainly from glycolysis: the oxidation of a glucose molecule yields two pyruvate molecules.
How much is the rate of decarboxylation reduced?
However, rate of decarboxylation is reduced by over 70% compared to the wild-type enzyme, as well as, once the multienzyme complex is assembled with the mutant pyruvate dehydrogenase, the PDC activity, which is reduced by over 85% compared to the wild-type complex.
Which group of dihydrolipoyl transacetylase is reductively acety?
the reductive acetylation of the lipoyl group of dihydrolipoyl transacetylase.
Where is the pyruvate dehydrogenase complex located?
In eukaryotes, the pyruvate dehydrogenase complex, like the enzymes for citric acid cycle and oxidation of fatty acids, is located in the mitochondrion, where is associated with the surface of the inner membrane facing the matrix. In prokaryotes, it is located in the cytosol.
What is the function of PDH?
PDH facilitates the use of carbohydrate to meet energy demands: when carbohydrate stores are depleted in mammals, PDH activity is downregulated to limit the use of glucose by oxidative phosphorylation. Fatty acids or ketone bodies are then used as an energy source in tissues such as heart and skeletal muscle.
What is the PDH pathway?
Pyruvate Dehydrogenase complex (PDH) connects the citric acid cycle and subsquent oxidative phosphorylation to the glycolysis, gluconeogenesis and lipid and amino acid metabolism pathways.
What is the key to measure endogenous PDH activity?
The key to measure endogenous PDH activity is to maintain the in vivo state of phosphorylation. Our extraction and immunocapture buffer have been formulated to inhibit specific and non-specific kinases and phosphatases to prevent unwanted PDH modifications during the sample preparation.
Which subunit of PDH is regulated by kinases?
The key regulatory subunit of PDH is the E1-alpha subunit (PDH1), modified by kinases at three serine phosphorylation sites to decrease activity.
Can PDH be analyzed ex vivo?
PDH activity can also be analyzed ex vivo following following PDH immunocapture and removal of endogenous kinases and phosphatases. Samples are then phosphorylated with recombinant PDK or dephosphorylated with recombinant PDP to determine (a) residual activity of the fully-phosphorylated PDH, (b) maximum activity of the fully dephosphorylated enzyme and (c) endogenous unmodified PDH activity.
What is pyruvate dehydrogenase?
Pyruvate dehydrogenase (PDH) deficiency is a congenital degenerative metabolic disease resulting from a mutation of the pyruvate dehydrogenase complex (PDC) located on the X chromosome. While defects have been identified in all 3 enzymes of the complex, the E1-α subunit is predominantly the culprit.
What is the name of the enzyme that converts pyruvate dehydrogenase into ace?
PDC consists of other enzymes, referred to as E2 and E3. Collectively E1-E3 transform pyruvate, NAD +, coenzyme A into acetyl-CoA, CO 2, and NADH. The conversion is crucial because acetyl-CoA may then be used in the citric acid cycle to carry out cellular respiration. To distinguish between this enzyme and the PDC, it is systematically called pyruvate dehydrogenase (acetyl-transferring) .
What enzyme catalyzes the reaction of pyruvate and a lipoamide to give the?
Pyruvate dehydrogenase is an enzyme that catalyzes the reaction of pyruvate and a lipoamide to give the acetylated dihydrolipoamide and carbon dioxide. The conversion requires the coenzyme thiamine pyrophosphate. Pyruvate dehydrogenase is usually encountered as a component, referred to as E1, of the pyruvate dehydrogenase complex (PDC).
What is the name of the enzyme that catalyzes the reaction of pyruvate and a?
Thiamine pyrophosphate (TPP) is shown in grey ball and stick form, two magnesium ions in purple undergoing metal ligation with the TPP, and two potassium ions in orange. Pyruvate dehydrogenase is an enzyme that catalyzes the reaction of pyruvate and a lipoamide to give the acetylated dihydrolipoamide and carbon dioxide.
What is PDBe-KB?
PDBe-KB provides an overview of all the structure information available in the PDB for Human pyruvate dehydrogenase (lipoamide) beta.
What enzyme is responsible for oxidation of pyruvate?
In bacteria, a form of pyruvate dehydrogenase (also called pyruvate oxidase, EC 1.2.2.2) exists that links the oxidation of pyruvate into acetate and carbon dioxide to the reduction of ferrocytochrome. In E. coli this enzyme is encoded by the pox B gene and the protein has a flavin cofactor.
What is the mechanism of activation of TPP coenzyme?
In terms of details, biochemical and structural data for E1 revealed a mechanism of activation of TPP coenzyme by forming the conserved hydrogen bond with glutamate residue (Glu59 in human E1) and by imposing a V-conformation that brings the N4’ atom of the aminopyrimidine to intramolecular hydrogen bonding with the thiazolium C2 atom. This unique combination of contacts and conformations of TPP leads to formation of the reactive C2-carbanion, eventually. After the cofactor TPP decarboxylates pyruvate, the acetyl portion becomes a hydroxyethyl derivative covalently attached to TPP.
Why do people with pyruvate dehydrogenase complex (PDC) deficiency?
This is because many people with pyruvate dehydrogenase complex (PDC) deficiency pass away before they have children. Genetic changes that are new in a person are called de novo. In some cases, people who have a pathogenic variant in PDHA1 inherited the genetic change from their mother.
What is the treatment for pyruvate dehydrogenase deficiency?
This can prevent immediate worsening of the disease. [1] [4] Treatment options typically include supplementing cofactors including carnitine, thiamine, and lipoic acid.
What is PDC in medical terms?
Pyruvate dehydrogenase complex (PDC) deficiency is suspected in people who have lactic acidosis or signs of early-onset neurological disease such as seizures, lethargy, and poor feeding. A doctor may wish to order more tests including: [1] Brain MRI to check for brain damage.
Why do females have a copy of PDHA1?
However, because females have another copy of the PDHA1 gene that is producing the enzyme of the pyruvate dehydrogenase complex , some females may have less severe symptoms of the disease. [1] In some cases, people who have a pathogenic variant in PDHA1 are the first people in the family with the genetic change. [5] .
How do you know if you have pyruvate dehydrogenase complex?
Signs that may be apparent in pregnancy include poor fetal weight gain and low levels of estriol in the mother’s urine. [4] Some babies with the disease may have brain abnormalities seen on ultrasound. [1] Babies with PDC deficiency may have low scores measuring a baby’s health after birth ( Apgar scores ). A low birth weight is common. Some features that may be characteristic of PDC deficiency include a narrow head, prominent forehead ( frontal bossing ), wide nasal bridge, long philtrum, and flared nostrils. However, these features are not present in all babies with PDC deficiency. [2]
What is PDC deficiency?
Pyruvate dehydrogenase complex (PDC) deficiency is a type of metabolic disease. This means that the body is not able to efficiently break down nutrients in food to be used for energy. Symptoms of PDC deficiency include signs of metabolic dysfunction such as extreme tiredness (lethargy), poor feeding, and rapid breathing ( tachypnea ). Other symptoms may include signs of neurological dysfunction such as developmental delay, periods of uncontrolled movements ( ataxia ), low muscle tone ( hypotonia ), abnormal eye movements, and seizures. Symptoms usually begin in infancy, but signs can first appear at birth or later in childhood. Symptoms may be especially apparent during times of illness, stress, or after meals with high amounts of carbohydrates. [1]
Why is PDC deficiency variable?
The signs and symptoms of PDC deficiency are variable because the amount of enzyme that is available to create energy varies in different people with the disease. [1]
What is pyruvate dehydrogenase?
Pyruvate dehydrogenase deficiency is characterized by the buildup of a chemical called lactic acid in the body and a variety of neurological problems . Signs and symptoms of this condition usually first appear shortly after birth, and they can vary widely among affected individuals. The most common feature is a potentially life-threatening buildup ...
What is the role of pyruvate dehydrogenase in the cell cycle?
This complex plays an important role in the pathways that convert the energy from food into a form that cells can use. The pyruvate dehydrogenase complex converts a molecule called pyruvate, which is formed from the breakdown of carbohydrates, into another molecule called acetyl-CoA. This conversion is essential to begin the series of chemical reactions that produce energy for cells.
What genes are involved in the E1 beta gene?
Mutations in the genes that provide instructions for E1 beta (the PDHB gene), the E2 enzyme (the DLAT gene), E3 binding protein (the PDHX gene), and pyruvate dehydrogenase phosphatase (the PDP1 gene) have been identified in people with this condition.
How do mutations affect the pyruvate dehydrogenase complex?
Although it is unclear how mutations in each of these genes affect the complex, reduced functioning of one component of the complex appears to impair the activity of the whole complex. As with PDHA1 gene mutations, changes in these other genes lead to a reduction of pyruvate dehydrogenase complex activity.
Why does a female have a pyruvate dehydrogenase deficiency similar?
However, many females with one altered copy of this gene have pyruvate dehydrogenase deficiency similar to affected males because the X chromosome with the normal copy of the PDHA1 gene is turned off through a process called X-inactivation.
What is the E1 enzyme?
The E1 enzyme, also called pyruvate dehydrogenase, is composed of four parts (subunits): two alpha subunits (called E1 al pha) and two beta subunits (called E1 beta). Mutations in the gene that provides instructions for making E1 alpha, the PDHA1 gene, are the most common cause of pyruvate dehydrogenase deficiency, ...
What proteins are involved in the function of a complex?
In addition, other proteins included in the complex ensure its proper function. One of these proteins, E3 binding protein , attaches E3 to the complex and provides the correct structure for the complex to perform its function. Other associated proteins control the activity of the complex: pyruvate dehydrogenase phosphatase turns on (activates) ...
What is the first enzyme in pyruvate dehydrogenase?
The first enzyme in this complex is called pyruvate dehydrogenase and removes the carboxylic acid group (decarboxylates) the molecule. The result of this reaction leaves a two-carbon molecule containing a methyl group and a carbonyl group.
What is pyruvate in biology?
Pyruvate Definition. Pyruvate is an important molecule that is present at the intersection of multiple biochemical pathways. It is commonly encountered as one of the end products of glycolysis, which is then transported to the mitochondria for participating the citric acid cycle. In the absence of oxygen, or when oxygen demand outstrips supply, ...
What is the conversion of G3P to pyruvic acid?
Thereafter, G3P is converted to pyruvic acid, which exists as its conjugate base at physiological concentration and pH. This process occurs through a set of five biochemical reactions, releasing two molecules of ATP and one molecule of NADH for each molecule of G3P.
Why is pyruvate fermented?
If aerobic respiration is not possible, pyruvate can be fermented to lactate in the cytoplasm to generate NADH and thereby increase the ATP availability for the cell. The enzyme involved in pyruvate fermentation can also catalyze the reverse reaction, forming pyruvate from lactate. This is particularly important in the cells of the liver where this is an essential process during the recovery period after exercise.
What is the name of the enzyme that catalyzes decarboxylation?
Within the matrix of the mitochondria, an important multi-enzyme complex called pyruvate dehydrogenase complex (PDC) catalyzes decarboxylation and oxidation reactions in order to generate acetyl coenzyme A (acetyl-CoA). The first enzyme in this complex is called pyruvate dehydrogenase and removes the carboxylic acid group (decarboxylates) ...
How many atoms are in pyruvic acid?
It contains three atoms that can act as hydrogen-bond donors and one atom that can be a hydrogen-bond acceptor. Like other keto acids, pyruvic acid can also tautomerize from its ketone form to its enol form, containing a double bond and an alcohol. This is particularly important in the last step of glycolysis.
What is the first step in glycolysis?
Glycolysis begins with the six-carbon monosaccharide – glucose. In the first few steps of this biochemical pathway, glucose undergoes phosphorylation and isomerization to produce fructose-6-phosphate. Another phosphorylation reaction facilitates the splitting of this hexose sugar into two 3-carbon molecules – glyceraldehyde phosphate (G3P) and dihydroxy acetone phosphate (DHAP). These initial steps require the input of energy and utilize two molecules of ATP for every molecule of glucose, but result in the major transformation of a hexose into two triose molecules.
What is the reaction of PDH and NADH?
The PDH oxidizes pyruvate into acetyl CoA and CO₂. This reaction generates NADH.
Which acid is conjuaged to PDH complex with an amide bond?
4. Lipoate/Lipoic Acid - conjuaged to PDH Complex with an amide bond.
How many cofactors does the PDH complex have?
The PDH complex uses three cofactors. What are they, and which subunit are they associated with?
Is PDH complex regulated allosterically?
The PDH complex is also regulated allosterically. Which molecules activate the complex, and which molecules inactivate it?
Can pyruvate diffuse through the membrane?
Pyruvate is charged, so it cannot diffuse through the inner membrane.
Is pyruvate a hydroxyethyl group?
2. Pyruvate is decarboxylated, and the 2 carbon hydroxy-ethyl group is transferred to TPP forming hydroxyethyl-TPP. The hydroxyethyl group is oxidized to form an acetyl group.
What is the enzyme that converts pyruvate into lactic acid?
Lactate dehydrogenase (LDH) is an enzyme found in most living organisms responsible for the conversion of pyruvate, the end product of glycolysis, into lactic acid. With this conversion, the molecule also uses a unit of the energy transferring molecule NADH, releasing the hydrogen to produce NAD+, allowing glycolysis to continue.
What is the function of lactate dehydrogenase?
Lactate dehydrogenase is present within all the cells of your body and works to maintain homeostasis in the absence of oxygen. When a person exercises hard, oxygen levels within muscle tissues drop quickly. In order for the muscle cells to keep functioning, they need to continue creating ATP.
Why do doctors use lactate dehydrogenase?
The lactate dehydrogenase test can be used to detect tissue damage. Further, because of the differentiation of different types of the enzyme, doctors can use a lactate dehydrogenase test to determine where and how much damage is taking place in the body.
How is pyruvate converted to lactic acid?
Instead of using the pyruvate in the Krebs cycle, the pyruvate is converted to lactic acid via lactate dehydrogenase. This process regenerates NAD +, which is needed to continue glycolysis. Continuing glycolysis produces a small amount of ATP, which allows the cell to survive.
How does glycolysis help muscles?
To continue functioning, the muscles must use the ATP created by the process of glycolysis. This process, to continue, needs electron carriers. Lactate dehydrogenase, in forming lactic acid, removes electrons from NADH to complete the process. In doing so, NAD + is created and can then be used in glycolysis to create more ATP. While the process produces far less ATP than the electron transport chain, it allows the cell to continue functioning without ample oxygen.
Why is NAD + necessary?
This conversion is necessary when a cell has little to no oxygen because NAD + is necessary to continue making ATP through glycolysis. The enzyme creates lactic acid as an end product, in a fermentation reaction. Lactic acid creates the feeling of your muscles “burning” when you exercise hard because it is building up in the cells. However, the true product of lactate dehydrogenase is more electron carriers, specifically NAD +.
Where is lactate dehydrogenase found?
In the human body, there are 5 different isoforms, or versions, of lactate dehydrogenase. These different versions are found in different body tissues, which can help doctors identify where the lactate dehydrogenase come from. For instance, LDH-1 (lactate dehydrogenase-1) is found in the heart, blood cells, and brain cells.

Overview
Pyruvate dehydrogenase is an enzyme that catalyzes the reaction of pyruvate and a lipoamide to give the acetylated dihydrolipoamide and carbon dioxide. The conversion requires the coenzyme thiamine pyrophosphate.
Pyruvate dehydrogenase is usually encountered as a component, referred to as E1, of the pyruvate dehydrogenase complex (PDC). PDC consists of other enzy…
Mechanism
The thiamine pyrophosphate (TPP) converts to an ylide by deprotonation. The ylide attack the ketone group of pyruvate. The resulting adduct decarboxylates. The resulting 1,3-dipole reductively acetylates lipoamide-E2.
In terms of details, biochemical and structural data for E1 revealed a mechanism of activation of TPP coenzyme by forming the conserved hydroge…
Structure
E1 is a multimeric protein. Mammalian E1s, including human E1, are tetrameric, composed of two α- and two β- subunits. Some bacterial E1s, including E1 from Escherichia coli, are composed of two similar subunits, each being as large as the sum of molecular masses of α- and β- subunits.
Active site
E1 has two catalytic sites, each providing thiamine pyrophosphate (TPP) and magnesium ion as cofactors. The α- subunit binds magnesium ion and pyrophosphate fragment while the β-subunit binds pyrimidine fragment of TPP, forming together a catalytic site at the interface of subunits.
The active site for pyruvate dehydrogenase (image created from PDB: 1NI4) hol…
Regulation
Phosphorylation of E1 by pyruvate dehydrogenase kinase (PDK) inactivates E1 and subsequently the entire complex. PDK is inhibited by dichloroacetic acid and pyruvate, resulting in a higher quantity of active, unphosphorylated PDH. Phosphorylation is reversed by pyruvate dehydrogenase phosphatase, which is stimulated by insulin, PEP, and AMP, but competitively inhibited by ATP, NADH, a…
Pathology
Pyruvate dehydrogenase is targeted by an autoantigen known as anti-mitochondrial antibodies (AMA), which results in progressive destruction of the small bile ducts of the liver, leading to primary biliary cirrhosis. These antibodies appear to recognize oxidized protein that has resulted from inflammatory immune responses. Some of these inflammatory responses could be related to gluten sensitivity as over 50% of the acute liver failure patients in one study exhibited a nonmit…
Related enzymes
In bacteria, a form of pyruvate dehydrogenase (also called pyruvate oxidase, EC 1.2.2.2) exists that links the oxidation of pyruvate into acetate and carbon dioxide to the reduction of ferrocytochrome. In E. coli this enzyme is encoded by the pox B gene and the protein has a flavin cofactor. This enzyme increases the efficiency of growth of E. coli under aerobic conditions.
See also
• Pyruvate dehydrogenase deficiency