What is the role of synaptotagmin in exocytosis?
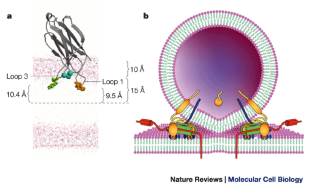
We proposed that synaptotagmin promotes synaptic vesicle insertion by the local buckling of the plasma membrane under the synaptic vesicle which is tethered to the plasma membrane by the SNARE complex.
Is synaptotagmin a calcium sensor?
Synaptotagmin: a Calcium Sensor on the Synaptic Vesicle Surface.
Is synaptotagmin a neurotransmitter?
Synaptotagmin 1 (Syt1) is a synaptic vesicle integral membrane protein that regulates neurotransmitter release by activating fast synchronous fusion and suppressing slower asynchronous release.
Is synaptotagmin a calcium-binding protein?
Synaptotagmin is the major calcium sensor for fast synaptic transmission that requires the synchronous fusion of synaptic vesicles. Synaptotagmin contains two calcium-binding domains: C2A and C2B.
Is Synaptotagmin a SNARE protein?
The core SNARE complex is formed by four α-helices contributed by synaptobrevin, syntaxin and SNAP-25, synaptotagmin serves as a calcium sensor and closely regulates the SNARE zipping....SNARE (protein)SNARE-fusion membrane complex proteinsIdentifiersOPM protein3hd7Membranome1985 more rows
Is vesicle docking calcium dependent?
A new model of synaptic plasticity emerges where calcium-dependent vesicular docking acts to enhance synaptic strength. This mechanism occurs over a variety of time domains, and it likely contributes to synaptic facilitation, post-tetanic potentiation, and depolarization-induced potentiation.
How do you pronounce Synaptotagmin?
0:011:07Pronunciation of the word(s) "Synaptotagmin". - YouTubeYouTubeStart of suggested clipEnd of suggested clipSe naturalmente senatur diament senna tú también senatur diament senatur diament se naturalmenteMoreSe naturalmente senatur diament senna tú también senatur diament senatur diament se naturalmente senna tú también senna tú también.
What is the function of synaptophysin?
It is present in neuroendocrine cells and in virtually all neurons in the brain and spinal cord that participate in synaptic transmission. It acts as a marker for neuroendocrine tumors, and its ubiquity at the synapse has led to the use of synaptophysin immunostaining for quantification of synapses.
Is Syntaxin a protein?
Syntaxins are a family of membrane-integrated proteins that are instrumental in exocytosis of vesicles. Syntaxin-1 is an essential component of the presynaptic exocytotic fusion machinery in the brain and interacts with several other proteins.
Where is Synaptotagmin located?
the synaptic vesiclesSynaptotagmin I is located in the synaptic vesicles and interacts with syntaxin, found on the plasma membrane (Chapman et al., 1995).
Is synaptobrevin a calcium sensor?
Synaptotagmin–1,−2, and −9 (Syt1, 2, 9) are the known Ca2+ sensors for fast, synchronous transmitter release in the millisecond time window following an AP (Südhof, 2014).
Does calcium bind to calmodulin?
Calmodulin is a low molecular weight, acidic, calcium binding protein which mediates the Ca2+ regulation of a wide range of physiological processes throughout eukaryotic organisms.
Is synaptobrevin a calcium sensor?
Synaptotagmin–1,−2, and −9 (Syt1, 2, 9) are the known Ca2+ sensors for fast, synchronous transmitter release in the millisecond time window following an AP (Südhof, 2014).
What is the function of synaptobrevin?
The small synaptic vesicle protein synaptophysin is considered as a marker protein for synapses during neuronal development. Another small synaptic vesicle protein, synaptobrevin, is now well accepted to play an important role for the function of synapses in being a key component of exocytosis.
Does calcium bind to calmodulin?
Calmodulin is a low molecular weight, acidic, calcium binding protein which mediates the Ca2+ regulation of a wide range of physiological processes throughout eukaryotic organisms.
What proteins does Complexin interact with?
Complexin's central region (amino acids 48–70) binds to the SNARE core as an anti-parallel α-helix, which attaches complexin to the SNARE complex. It interacts selectively with the ternary SNARE complex but not with monomeric SNARE proteins. Complexin binds to the groove between the synaptobrevin and syntaxin helices.
What are the functions of synaptotagmins?
Synaptotagmin I facilitates synaptic vesicle membrane fusion with the presynaptic membrane, a function that shares striking similarity to Fer-1 function (Brose et al., 1992). Synaptotagmin I is located in the synaptic vesicles and interacts with syntaxin, found on the plasma membrane (Chapman et al., 1995). In the presence of calcium, the C2A domain of synaptotagmin I binds syntaxin with a high affinity and also binds negatively charged phospholipids with increased affinity (Brose et al., 1992; Perin et al., 1990 ). The Ca 2+ binding loops of the C2A and C2B domains of synaptotagmin I insert into the lipid bilayer (Bai et al., 2002 ). Synaptotagmin I has the spatial opportunity to mediate vesicular–plasma membrane fusion while bound to the vesicular membrane and interacting with syntaxin in the presence of calcium. Thus, synaptotagmin I is thought to regulate timing of membrane fusion through its calcium-sensing ability and its ability to promote membrane fusion through Ca 2+ loop insertion into the membrane, causing a local disruption ( Peuvot et al., 1999). One theory of membrane coalescence suggests that the disruption of the lipid bilayer by the angled insertion of calcium-sensing proteins induces membrane fusion (Peuvot et al., 1999 ).
What are the genes of the synaptotagmin family?
The mammalian synaptotagmin family includes 16 genes , whereas the Caenorhabditis elegans and Drosophila melanogaster genomes encode eight and seven synaptotagmin genes, respectively. In Drosophila and mice, the expression of most synaptotagmin isoforms is restricted to the nervous system. Despite the similarity in their principal domain organization, different members of the synaptotagmin family display distinct expression patterns, subcellular localizations, and biochemical properties. Of the 16 synaptotagmins identified in the mouse genome, four isoforms, synaptotagmins-1, -2, -9, and -12, are localized on the synaptic vesicles, whereas the remaining isoforms are likely to be targeted to the various intracellular membrane compartments. The prototypical member of the synaptotagmin family, synaptotagmin-1, was originally identified in a monoclonal antibody screen for synaptic vesicle proteins. Subsequent genetic and functional studies showed that synaptotagmin-1 acts as a Ca2+ sensor for fast synaptic vesicle exocytosis. In Drosophila and mice, deletion of the synaptotagmin-1 gene results in lethal phenotypes associated with the specific loss of fast (synchronous) Ca 2+-triggered neurotransmitter release (Figure 2 ); whereas the mutations in the mouse synaptotagmin-1 gene that alter overall synaptotagmin-1 Ca 2+ -binding affinity produce a similar shift in a Ca 2+ sensitivity of transmitter release. Recent studies showed that, similar to synaptotagmin-1, synaptotagmins-2 and -9 act as Ca2+ sensors for fast synaptic vesicle exocytosis. However, the functional roles of most nonsynaptic vesicle synaptotagmin isoforms remain controversial.
What are the roles of snare proteins in fusion?
While the SNARE proteins and synaptotagmins are both implicated in the kinetics of membrane fusion and thus in the fusion event itself there are many other accessory proteins that likely regulate the placement of the fusion site, the assembly of fusion complexes and other proteins may also participate directly in the fusion event. Thus, proteins showing phenotypes in docking or priming of vesicle fusion may not be excluded from playing a role in later stages. Indeed, synaptotagmin-1 has now been proposed to play a role in docking, fusion, and in the post fusion opening of the fusion pore (collapse of the vesicle). Finally, while synaptic vesicle fusion is Ca2+ -dependent many other fusion events are constitutive and do not involve a Ca 2+ trigger, but yet are still likely to require membrane identity selection and membrane priming by accessory proteins of the SNARE complex in an analogous manner to C2 domains.
Why do mice die from synaptotagmin knockout?
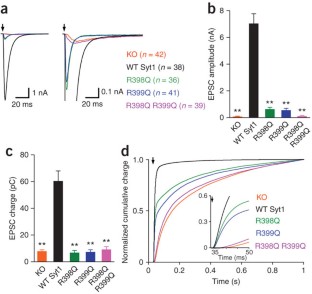
Synaptotagmin knockout mice homozygous for the missing gene die at birth because they cannot breathe well enough, so experimenters could not study synaptotagmin knockout synapses in vivo. They instead made hippocampal cell cultures from control (wild-type) and embryonic synaptotagmin knockout mice, permitted these neuronal cultures to form synapses and mature their synaptic contacts for 2 weeks in vitro, and compared synaptic transmission in control and synaptotagmin knockout synapses using whole-cell patch clamp techniques.
What is the slope of a control synapse?
Control synapses ( WT) have a calcium-release relationship with a slope of 3.5 ( black line ). A single point mutation in the C2B calcium-binding domain maintains the same relationship (slope=3.6; red line ), but shifts the sensitivity of neurotransmitter release to calcium to the right, meaning that more calcium is required to trigger the same amount of transmitter release. A mutation that removes the entire C2B calcium-binding domain from synaptotagmin reduces calcium-triggered transitter release, and changes the slope of the calcium-release relationship (slope=1.6; blue line ).
What is the active zone of a synapse?
These steps occur at a dense protein network called the active zone of a synapse. The active zone is attached to the presynaptic plasma membrane and forms highly organized sites for the release of neurotransmitters ( Fig. 15.8, Couteaux and Pecot-Dechavassine, 1970; Südhof, 2012 ). Detailed tomographic electron microscopy allows a more precise look at the association of vesicles and plasma membrane at the active zone. At frog neuromuscular junctions an electron-dense “fuzz” adjacent to the presynaptic plasma membrane is a highly ordered structure—a lattice of proteins that connect the vesicles to a cytoskeleton, to one another, and to the plasma membrane ( Harlow et al., 2001) ( Fig. 15.8 ). In typical synapses of the vertebrate brain, active zones form a hexagonal grid with depressions for docking and fusion of synaptic vesicles ( Fernandez-Busnadiego et al., 2010; Limbach et al., 2011; Pfenninger et al., 1972 ). In other synapses, such as photoreceptors, hair cells, and many insect synapses, the structures are more elaborate and include ribbons, dense bodies, or T-bars that extend into the cytoplasm and appear to have a special relationship with nearby vesicles ( Zhai and Bellen, 2004 ).
Which calcium sensor is responsible for vesicle release?
Taken together, the above studies strongly support the hypothesis that synaptotagmin is the primary calcium sensor for fast calcium-triggered vesicle release. However, there is also evidence that other calcium-binding proteins in the nerve terminal can act as secondary calcium sensors that increase the probability of neurotransmitter release ( Sun et al., 2007 ), though they may be slower-acting and/or have a higher affinity for calcium than synaptotagmin . The identity of these secondary calcium sensors is still debated, but they have been proposed to selectively trigger slower asynchronous neurotransmitter release (for example, Doc2 or synatotagmin-7; Wen et al., 2010; Yao et al., 2011 ), spontaneous neurotransmitter release (Doc2; Groffen et al., 2010 ), and/or to increase the probability of neurotransmitter release during short-term plasticity (synaptotagmin-7; Jackman et al., 2016; see Chapter 14 ).
What is the synaptotagmin 1?
Synaptotagmin 1 is comprised of a short, glycosylated lumenal stretch, a single transmembrane domain and two cytoplasmic Ca2+ binding C2 domains, termed C2A and C2B that lack classical endocytosis signals including YxxØ and [DE]xxxL [LIM] motifs. From: European Journal of Cell Biology, 2012.
What is the function of the NCS-1?
The neuronal calcium sensor (NCS) or frequenin family of calcium-binding proteins is made up of candidates for transducing calcium signals during exocytosis that are just beginning to be studied in detail. Overexpression of NCS-1 in AtT-20 cells, for example, has complex effects, blunting secretagogue stimulation of ACTH release in intact cells, yet enhancing calcium- and GTP-gamma-S-induced secretion from permeabilized cells [137] and, thus, possibly representing the link between GTP-binding proteins and calcium in the regulation of exocytosis by nonhydrolyzable GTP analogs reported in mast cells and AtT-20 cells. Frequenin's involvement in exocytosis at the neuromuscular junction appears to be specific for calcium influx through N-type VSCCs, suggesting that this calcium-binding protein may regulate calcium at very specific sites of exocytosis corresponding to calcium channel-vesicle nanodomains [138 ]. Coscaffolding of frequenin with N-type VSCCs within nerve terminals may explain the observation that both NCS and frequenin demonstrate inhibition of exocytosis at high concentrations, a hallmark of effects on cellular processes upon introduction of high concentrations of scaffolding and scaffolded proteins into many cell types [ 139 ].
What is the Ca2+ sensor?
There is substantial evidence that the Ca 2+ sensor for fast neurotransmission is a Ca 2+-binding protein known generically as synaptotagmin. Various isotypes of synaptotagmin, most generally synaptotagmin 1 but also synaptotagmins 2 and 9, can act as a trigger for rapid transmission, as indicated by experiments using knockout mice. There are two main theories about how synaptotagmin works: by unleashing the SNARE machinery by removal of a tonic inhibitory brake or by adding a fusion-promoting influence, derived from Ca2+/syt 1 binding to membrane phospholipids, to that already provided by preformed SNARE complexes.
What is the role of synaptic vesicle fusion?
Synaptic vesicle fusion, which is required for the release of neurotransmitters, has fascinated us for many years because of its unusually fast time scales (submicroseconds) and the tight regulation by Ca2+. There are several key players other than SNAREs such as synaptotagmin 1, complexin, and Munc18, which interact with SNAREs in an orchestrated fashion to induce this remarkable event. Dissection of functions of individual proteins and their interplay with SNAREs should hold the key to the understanding of mechanism.
What phospholipids bind to the top loops?
In all phospholipid-binding C 2 -domains, phospholipids bind exclusively to the top loops similar to Ca 2+ -binding. In spite of this similarity, however, the mechanism of phospholipid binding and the phospholipid specificity vary greatly among C 2 -domains. Some C 2 -domains (such as both C 2-domains of synaptotagmin 1) bind promiscuously negatively charged residues [6,20 ], whereas other (such as the C 2-domain of cytoplasmic phospholipase A2) bind neutral lipids [25 ]. Both hydrophobic and electrostatic interactions contribute to phospholipid binding but to different degrees in the various C 2 -domains (see e.g. [ 25,26 ]). The contribution of different types of interactions has been described in detail for the double C 2 -domain fragment of synaptotagmin 1 in which both C 2 -domains contribute to the overall interaction [ 27,28 ]. Here, each C 2-domain separately participates in three types of interactions with the phospholipid bilayer, Ca2+ -mediated binding, hydrophobic attachment, and electrostatic interactions via positively charged residues.
What is the function of C2A domains?
This is true even for C2 -domains that do not bind Ca 2+; in fact, the function of most Ca 2+ -independent C 2-domains appears to be to attach their resident proteins to phospholipid membranes [21–24 ]. Ca 2+ -independent phospholipid binding is possibly best illustrated by the C 2-domain of PTEN, a tumor suppressor gene that is a phosphatase for the lipid phosphatidylinositol 3,4,5-trisphosphate [21,22 ]. The C 2-domain of PTEN positions its catalytic domain on top of the substrate. The C2-domain not only recruits PTEN to the membrane, it also orients the catalytic domain with respect to the membrane substrate. Similar functions have been ascribed to the N-terminal C2 -domains of novel PKCs whose structures have been solved [ 23,24 ]. However, although significant evidence exists that phospholipid binding may be an even more general property of C 2 -domains than Ca 2+ -binding, it seems likely that not all C 2 -domains bind phospholipids.
What is the calcium sensor for exocytosis?
Several other calcium-binding proteins have been investigated as calcium sensors for exocytosis in a variety of secretory cells. The calcium-activated protein for secretion (CAPS) is an ubiquitous cytosolic protein that nevertheless binds LDCVs, but not SSVs, in a highly specific and calcium-dependent fashion [ 132 ]. Its ablation in Drosophila demonstrates that it is essential for LDCV exocytosis [ 133 ]. CAPS is present in all secretory cells that exhibit calcium-regulated secretion [ 134] and, interestingly, is absent from the parotid gland [ 61 ]. A puzzling aspect of CAPS involvement in LDCV exocytosis is its relatively low affinity (about 250 μ M) for calcium [ 132 ], since the calcium threshold for exocytosis in, for example, chromaffin and PC12 cells is less than 10 μM [ 135 ]. However, calcium affinities in vitro and in vivo may easily be discrepant, given both the possibility for cooperativity with other calcium-binding proteins in the exocytotic process, and the need to define very carefully the pool of docked vesicles contributing to secretion in a given experimental paradigm, only a small fraction of which is “readily releasable” [ 58,136 ].
Neurotransmitter release
Constance Hammond, ... Michael Seagar, in Cellular and Molecular Neurophysiology (Fourth Edition), 2015
Synaptotagmins
Apart from synaptotagmin-1, three additional synaptotagmin isoforms are localized on the synaptic vesicles. Two of these isoforms, synaptotagmins-2 and -9 (also called synaptotagmin-5), contain functional Ca 2+ -binding sites in their C 2 domains and display Ca 2+ -dependent phospholipid and SNARE-binding activities.
Organelles
Samples were analyzed using SDS-PAGE and immunoblotting. Antibodies were raised against the complete p65 protein or amino acids 220–234 of the p65 protein sequence. Antibodies to the enzyme mannosidase II and the peripheral Golgi membrane protein GM130 were purchased.
RNA Editing
Most A‐to‐I RNA editing, which causes recoding, occurs in genes that affect neuronal signaling. Drosophila synaptotagmin I ( dsytI) is the Ca 2+ sensor for neurotransmitter release ( Yoshihara and Littleton, 2002) and a target for ADAR ( Hoopengardner et al., 2003 ), with one exon containing four A‐to‐I RNA editing sites.
C2 Domains and Membrane Fusion
Spontaneous release has long been regarded as some oddity, which may be the result of an accidental trigger of the release machinery.
What is the function of CNTs?
The LCs of CNTs are proteolytic enzymes that are solely responsible for the toxin action in inhibiting neurotransmitter release. Unlike other neurotoxins that function as channel or receptor blockers, the enzymatic nature of CNTs allows for elimination of many targets by a single CNT molecule. It is thus conceivable that a single CNT molecule suffices to inactivate a nerve terminal, which may be one of the reasons why the CNTs are by far the most potent of all known natural toxins.
What are the proteins that are expressed in IHC ribbon synapses?
IHC ribbon synapses share the expression of several proteins of the AZs (e.g., bassoon, CAST/ELKS, piccolo/piccolino, RIMs, RIM-BP, see above and Fig. 1) as well as the vesicle proteins rab3a, rab3c, and CSPβ with conventional synapses. However, some aspects of their molecular machinery seem quite unconventional. For example, instead of using VGLUT1 or 2 as other glutamatergic synapses, they use VGLUT3 ( Obholzer et al., 2008; Ruel et al., 2008; Seal et al., 2008 ). Moreover, neither synapsins, synaptophysin, synaptogyrin ( Reisinger et al., 2011; Safieddine and Wenthold, 1999 ), priming proteins of the Munc13 and CAPS family ( Vogl et al., 2015 ), the Ca 2+ sensors of neuronal SV fusion synaptotagmins 1 or 2 ( Beurg et al., 2010; Reisinger et al., 2011 ), nor complexins ( Strenzke et al., 2009) seem to operate at the mature IHC synapse. The probably most striking and still controversional notion on the molecular physiology of IHC exocytosis came from a study that did not find evidence for a function of the neuronal SNAREs SNAP-25, syntaxin 1 and synaptobrevins 1–3 ( Nouvian et al., 2011 ). While providing evidence for absence is challenging, this study employed clostridial neurotoxins of well-controlled activity and abundance in the recorded IHCs, knock-out mice, as well as immunohistochemistry and the results collectively suggested lack or functional irrelevance of neuronal SNARE proteins at the IHC synapse. Nonetheless, expression of neuronal SNAREs has been indicated by mRNA analysis ( Nouvian et al., 2011; Safieddine and Wenthold, 1999; Shen et al., 2015 ), co-immunoprecipitation with otoferlin from the organ of Corti ( Roux et al., 2006 ), and immunolabeling ( Safieddine and Wenthold, 1999 ).
How does CNT acidify?
As mentioned previously, the exact nature of CNT uptake compartments is unknown, but there is general consensus that these compartments acidify by the activity of the vacuolar proton pump after internalization. Like other bacterial toxins that translocate enzymatically active domains into the cytoplasm, CNTs undergo a major pH-dependent structural rearrangement that predominantly involves the H N C domain (translocation domain). As a result, the toxin is converted from a water-soluble conformation into a conformation in which hydrophobic segments are exposed on the surface. It is assumed that in this conformation the toxin can permeate the lipid bilayer of the endocytic compartment. Evidence for a mechanism involving the formation of hydrophilic pores has been provided by studies showing that the H N C domains of TeNT and BoNTs form ion channels in artificial and biological membranes at low pH. These channels probably operate as protein-translocating channels that transfer the catalytic domain across the vesicle membrane into the cytosol of the nerve terminal. Alternatively, the channel-induced permeability changes could lead to osmotic lysis of the CNT-containing organelles, thereby releasing the toxin into the cytosol of the nerve terminal. In any case, the released toxin is not active unless the catalytic domain refolds in the neutral environment of the cytosol, and the interchain disulfide bond is completely reduced.
What are complex gangliosides?
Complex gangliosides, a class of glycosphingolipids that are particularly abundant in the outer leaflet of nerve cell membranes, have long been recognized to function as receptors for CNT-HCs. Later, the existence of two classes of binding sites distinguished by different affinities and protease-sensitivities 86,87 led to a dual-receptor concept: complex gangliosides first accumulate CNTs on the plasma membrane surface before protein receptors subsequently mediate their endocytosis, with a different protein receptor being recognized by each BoNT. 22,88,89 Such a dual-receptor binding process could account for the extraordinary binding affinity and specificity of CNTs.
How are CNTs internalized?
Once bound to the surface of peripheral nerve terminals, CNTs are internalized by clathrin-mediated endocytosis into acidifying vesicular compartments. For this process, the H C C domains appear to be sufficient. For some CNTs, it has been shown that high-frequency stimulation of neurotransmitter release increases toxin internalization, indicating that CNTs exploit the endocytotic pathway of synaptic vesicles. This notion is supported by the fact that both synaptotagmins and SV2s serving as coreceptors for BoNTs are synaptic vesicle proteins. However, the exact nature of the toxin-carrying endocytotic compartments and their relation to early and recycling endosomes and to recycling synaptic vesicles is unclear. Furthermore, the reasons for the different intracellular pathways taken by BoNTs and TeNT have remained elusive. BoNTs largely remain within the nerve terminal they invade, a feature that has been exploited in their clinical use as safe drugs for blocking muscle excitation in a highly localized fashion. In contrast, TeNT-containing vesicles are transported retrogradely along the axon toward the dendrites of the motor neurons. Here, the toxin is released by postsynaptic exocytosis (transcytosis) and subsequently endocytosed by the nerve terminals of glycinergic interneurons that are then inhibited. What is the reason for this difference? It is possible that TeNT and BoNTs are internalized by different organelles or differentially sorted after endocytosis. Alternatively, the toxins may share a common entry pathway but TeNT then generates a signal that triggers vesicle binding to microtubule-dependent motors involved in retrograde transport, thus separating it from BoNT-containing vesicles that would remain inside the presynaptic terminal.
SUMMARY
Synaptotagmin-I (syt) is a Ca 2+ sensor that triggers synchronous neurotransmitter release. The first documented biochemical property of syt was its ability to aggregate membranes in response to Ca 2+. However, the mechanism and function of syt-mediated membrane aggregation are poorly understood.
INTRODUCTION
Communication between neurons relies on Ca 2+ -triggered exocytosis of synaptic vesicles. Fusion of synaptic vesicles with the plasma membrane is mediated by soluble NSF attachment protein receptors (SNAREs). The Ca 2+ sensitivity of this process is mainly conferred by syt, a vesicle protein that harbors tandem C2 domains, C2A and C2B.
RESULTS
Increases in the turbidity of vesicle suspensions are routinely used as an index of vesicle aggregation 22. We used this method to characterize syt-mediated vesicle aggregation by monitoring changes in turbidity over time after sequential addition of the C2AB domain of syt, Ca 2+ and excess EGTA to chelate Ca 2+ ( Fig. 1 ).
DISCUSSION
In the present study, we characterize the mechanism of syt-mediated membrane aggregation. Our data support a model in which syt-mediated aggregation relies on both syt-membrane interactions and intermolecular interactions.
METHODS
Synthetic 1,2-dioleoyl-sn-glycero-3-phospho-l-serine (phosphatidylserine, PS), 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (phosphatidylcholine, PC), 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine (phosphatidylethanolamine, PE), 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N- (biotinyl) (18:1 Biotin-PE), 1,2-dipalmitoyl-sn-glycero-3-phospho-ethanolamine-N- (7-nitro-2-1,3-benzoxadiazol-4-yl) (NBD-PE) and N- (lissamine rhodamine B sulfonyl)-1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine (rhodamine-PE) were purchased from Avanti Polar Lipids.
ACKNOWLEDGMENTS
We thank members of the Chapman laboratory for their critical discussions and comments during this study.
REFERENCES FOR METHODS
1. Hui E, Johnson CP, Yao J, Dunning FM, Chapman ER. Synaptotagmin-mediated bending of the target membrane is a critical step in Ca2+-regulated fusion. Cell. 2009;138:709–21. [ PMC free article] [ PubMed]
What is the function of synaptotagmin 1?
Synaptotagmin 1 is crucial for rapid Ca 2+ -evoked transmitter release by controlling SNARE function ( 4, 31, 32, 39, 45 ). Upon Ca 2+ binding to the C2 domains, synaptotagmin penetrates the plasma membrane containing anionic phospholipids such as phosphotidylserine ( 31, 32 ). The C2 domains also mediate Ca 2+ -independent and Ca 2+ -dependent interactions with syntaxin 1 and SNAP 25. Evidence suggests that Ca 2+ -dependent membrane binding by synaptotagmin is itself insufficient to trigger fusion but that it also requires Ca 2+ -dependent SNARE binding by synaptotagmin. An additional regulatory protein, complexin 1, also coordinates synaptotagmin function. Complexin 1 was originally thought to clamp SNARE complexes at a prefusion step, thereby inhibiting exocytosis ( 15, 60 ). Upon elevation of intracellular Ca 2+, complexin 1 was proposed to be displaced from the SNARE complex by synaptotagmin 1, in turn allowing the final stages of neurotransmitter release to commence ( 15 ). More recent studies have shown that, in addition to this clamping activity, complexin 1 also directly facilitates the final stages of exocytosis ( 48, 52 ). We recently reported that acinar cells express complexin 2 and further found that introduction of recombinant complexin 2 into permeabilized acini inhibited Ca 2+ -dependent exocytosis ( 8 ). Moreover, we showed that complexin 2 interacts with VAMP-2-positive granules. Taken together with the present results, it is conceivable that VAMP-2 ZGs together with synaptotagmin 1 and complexin 2 function in the early peak phase of the secretory response analogous to their concerted role in synaptic vesicle exocytosis. Clearly, however, the kinetics of ZG exocytosis are much slower than synaptic vesicles likely owing to differences in SNARE isoforms, Ca 2+ release kinetics, and the ∼50-fold larger diameter of ZGs.
Where is synaptotagmin 1 found?
Although originally described as a neuronal specific isoform, Musch et al. ( 36) reported that synaptotagmin 1 is also present in intestinal epithelial cells. Synaptotagmin 1 was highly localized to the apical membrane of enterocytes in the small intestine and was likewise present in a colon carcinoma cell line (Caco-2BBe) ( 36, 37 ). Expression of synaptotagmin 1 was confirmed by RT-PCR, immunoblotting, small interfering RNA (siRNA) studies and by direct sequencing of the immunoprecipitated protein. Although a specific role for synaptotagmin 1 in exocytosis was not investigated, the protein was shown to regulate cAMP- and Ca 2+ -induced endocytosis of the sodium/hydrogen exchanger 3 (NHE3) ( 36 ).
What are synaptotagmins made of?
Synaptotagmins are a family of membrane proteins composed of 15 known isoforms distributed widely in neuronal and nonneuronal tissues (for comprehensive reviews see Refs. 4, 39, 45 ). Family members have the same basic structure consisting of a short NH 2 -terminal intravesicular domain followed by a transmembrane domain and a large COOH-terminal cytoplasmic segment that contains two C2 domains: C2A and C2B. These C2 domains are found in tandem and provide the only homology shared among family members. The C2 domains function as Ca 2+ binding sites that mediate Ca 2+ -dependent interactions of synaptotagmin with other molecules including phospholipids and soluble N -ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins ( 31, 32 ). The C2A domain binds three and C2B binds two Ca 2+ ions. Although all isoforms have C2 domains, only eight show Ca 2+ -dependent phospholipid binding activity: 1, 2, 3, 5, 6, 7, 9, and 10. Additionally, the affinity for Ca 2+ is in the micromolar range but differs between isoforms; for example, the ubiquitously expressed synaptotagmin 7 has a higher affinity for Ca 2+ than the neuronal synaptotagmin 1. The absence of Ca 2+ -binding activity in other isoforms is due to one or more point mutations in the five aspartic acid residues that coordinate Ca 2+ -binding in each of the C2 domains. The binding affinity for phospholipids and the SNARE protein syntaxin also differs among family members. In vitro, synaptotagmins 1, 2, and 5 bind phospholipids at low micromolar Ca 2+ concentrations and syntaxins at higher concentrations, whereas synaptotagmins 3 and 7 bind both phospholipids and syntaxins at low micromolar Ca 2+ concentrations.
How are synaptotagmin 1 granules recruited?
Synaptotagmin 1-positive granules are recruited to the apical plasma membrane of acini upon stimulation with CCK-8. A: isolated acini were treated as control or with 100 pM CCK-8 for 5 min at 37°C and then incubated for 2 h at 4°C with anti-synaptotagmin 1 (1:20) to label externalized antigen. Cells were then fixed in 2% formaldehyde and immunoreactivity was detected postfixation, by use of Alexa Fluor 488-conjugated anti-rabbit IgG (1:250). Nuclei were labeled with DAPI. Each image is a reconstructed z -series obtained by confocal microscopy. Bars, 7 μM. All images are representative of multiple determinations performed on at least 3 separate tissue preparations. B: quantitative analysis measuring the volume of synaptotagmin 1 immunoreactivity in acini acquired from multiple reconstructed z -series images of single acini from 3 separate tissue preparations. Data are means and SE ( n = 10 for each experimental condition).
What is the role of Ca 2+ in secretion?
Secretagogue-induced changes in intracellular Ca 2+ play a pivotal role in secretion in pancreatic acini yet the molecules that respond to Ca 2+ are uncertain. Zymogen granule (ZG) exocytosis is regulated by soluble N -ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complexes. In nerve and endocrine cells, Ca 2+ -stimulated exocytosis is regulated by the SNARE-associated family of proteins termed synaptotagmins. This study examined a potential role for synaptotagmins in acinar secretion. RT-PCR revealed that synaptotagmin isoforms 1, 3, 6, and 7 are present in isolated acini. Immunoblotting and immunofluorescence using three different antibodies demonstrated synaptotagmin 1 immunoreactivity in apical cytoplasm and ZG fractions of acini, where it colocalized with vesicle-associated membrane protein 2. Synaptotagmin 3 immunoreactivity was detected in membrane fractions and colocalized with an endolysosomal marker. A potential functional role for synaptotagmin 1 in secretion was indicated by results that introduction of synaptotagmin 1 C2AB domain into permeabilized acini inhibited Ca 2+ -dependent exocytosis by 35%. In contrast, constructs of synaptotagmin 3 had no effect. Confirmation of these findings was achieved by incubating intact acini with an antibody specific to the intraluminal domain of synaptotagmin 1, which is externalized following exocytosis. Externalized synaptotagmin 1 was detected exclusively along the apical membrane. Treatment with CCK-8 (100 pM, 5 min) enhanced immunoreactivity by fourfold, demonstrating that synaptotagmin is inserted into the apical membrane during ZG fusion. Collectively, these data indicate that acini express synaptotagmin 1 and support that it plays a functional role in secretion whereas synaptotagmin 3 has an alternative role in endolysosomal membrane trafficking.
What is the purified protein of synaptotagmin 1 and 3?
Glutathione S -transferase fusion proteins of synaptotagmin 1 and 3 were purified by glutathione affinity chromatography and released from the beads by thrombin cleavage as previously described ( 18 ).
Which synaptotagmin binds to AP-2?
The C2B domain of synaptotagmin 1 binds to AP-2 ( 9) in a Ca 2+ -independent manner ( 56 ). Synaptotagmins 1 through 7 interact with AP-2 with high affinity ( 29, 61) and binding of synaptotagmin and AP-2 is stimulated by peptides with a tyrosine-based endocytic sorting motif ( 22 ).