What is the valence electron configuration of cerium?
May 01, 2020 · What is the ground state electron configuration for cesium? Caesium atoms have 55 electrons and the shell structure is 2.8. 18.18. 8.1. The ground state electron configuration of ground state gaseous neutral caesium is [Xe]. Click to see full answer.
How to find electron configuration?
Cesium. Full electron configuration of cesium: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 6s1. xenon ← cesium → barium.
How many electrons are in an atom of cesium?
Aug 03, 2019 · In the case of Cesium the abbreviated electron configuration is [Xe] 6s1. Nevertheless, check the complete configuration and other interesting facts about Cesium that most people don't know. Cesium Overview Cesium Complete Electron Configuration 1s2 2s2 2p6 3s2 3p6 4 s2 3 d10 4 p6 5 s2 4 d10 5 p6 6 s1 Abbreviated Electron Configuration [Xe] 6s1 …
What element is 1s22s22p63s23p64s23d104p5?
Mar 12, 2022 · The electron configuration of cesium is 1s2 2s2 2p6 3s2 3p6 4s2. The 2s electrons are in the lowest energy level, so they are of particular interest.

How do you write the electron configuration for cesium?
What element has the electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d6?
Which electron configuration represents a cesium atom in ground state?
What is ground state electron configuration?
What element is 1s2 2s2 2p6 3s2 3p6 3d10 4s2?
| A | B |
|---|---|
| copper | 1s2 2s2 2p6 3s2 3p6 4s1 3d 10 ! |
| bromine | 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5 |
| silver | 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s1 4d10 ! |
| lead | 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p2 |
What element is 1s2 2s2 2p6 3s2 3p6 4s2 3d3?
What is unstable electronic configuration?
What is the symbol of cesium?
What is the electronic configuration of fluorine?
Cesium(Cs) Electron Configuration Through Orbit
Electron Configuration of Cesium(Cs) Through Orbital
- Atomic energy levels are subdivided into sub-energy levels. These sub-energy levels are called orbital. The sub energy levels are expressed by ‘l’. The value of ‘l’ is from 0 to (n – 1). The sub-energy levels are known as s, p, d, f. Determining the value of ‘l’ for different energy levels is- 1. If n = 1, (n – 1) = (1–1) = 0 Therefore, the orbital number of ‘l’ is 1; And the orbital is 1s. 2. If n = 2, (n …
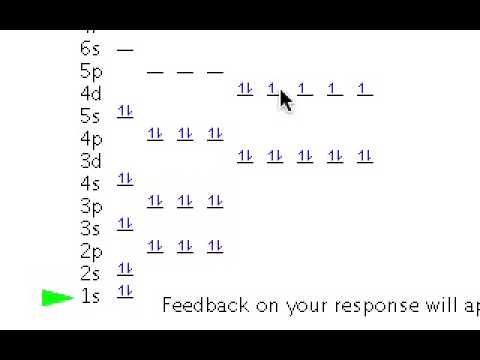
How to Write The Orbital Diagram For Cesium(Cs)?
- To create an orbital diagram of an atom, you first need to know Hund’s principle and Pauli’s exclusion principle. Hund’s principle is that electrons in different orbitals with the same energy would be positioned in such a way that they could be in the unpaired state of maximum number and the spin of the unpaired electrons will be one-way. And Pauli’s exclusion principle is that the …
Cesium Ion(Cs+) Electron Configuration
- Ground state electron configuration of cesium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 6s1. This electron configuration shows that the last shell of cesium has only an electron. Therefore, the valence electrons of cesium are one. The elements that have 1, 2, or 3 electrons in the last shell donate the electrons in the last shell durin…
FAQs
- What is the symbol for cesium? Ans:The symbol for cesium is ‘Cs’. How many electrons does cesium have? Ans:55 electrons. How do you write the electron configuration for cesium? Ans: Cesium electron configuration is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 6s1. How many valence electrons does cesium have? Ans:One valence electrons. What is the valency of c…