The elements of the periodic table sorted by ionization energy
| The chemical elements of the periodic ch ... | Ionization Energy | Name chemical element | Symbol | Atomic number |
| - Name alphabetically | 3,8939 | Cesium | Cs | 55 |
| - Atomic number | 4,0727 | Francium | Fr | 87 |
| - Symbol | 4,1771 | Rubidium | Rb | 37 |
| - Atomic Mass | 4,3407 | Potassium | K | 19 |
What trend in ionization energy occurs across a period on the periodic table?
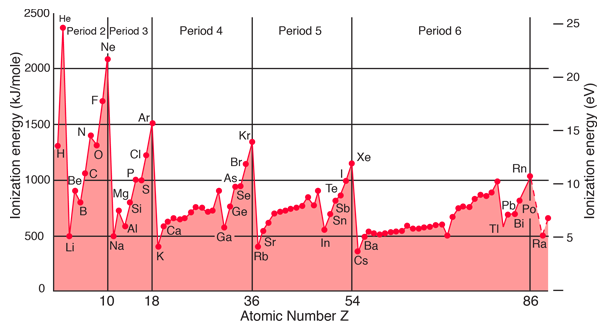
Ionization energy exhibits periodicity on the periodic table. The general trend is for ionization energy to increase moving from left to right across an element period. Moving left to right across a period, atomic radius decreases, so electrons are more attracted to the (closer) nucleus.
How to calculate the first ionization energy?
How to Calculate Ionization Energy. Ionization potential for hydrogen can be calculated using the following equation: E = hcRH (1/n 2 ), where. E is energy of the electron (or the amount of energy it takes to remove the electron, ionization energy) h is Planck’s constant = 6.626 * 10 -34 Js (joules*seconds)
What is the equation for ionization energy?
eV /atom = −2.18× 10−18 × n2Z2. . J /atom. Ionization energy for the removal of an electron from a neutral atom can be calculated, by substituting, the orbit number of the electron before transition as ‘n 1 ‘ and orbit number of the electron after transition as ‘∞' ( infinity) as ‘n 2 ‘ in Bohr’s energy equation.
How do you find ionization energy?
How to Find Ionization Energy Using a Qualitative Application of Coulomb's Law
- Steps to Find Ionization Energy. Step 1: Determine the effective nuclear charge for each element. ...
- Vocabulary and Equations for Finding Ionization Energy. ...
- Example Problem 1 - Estimating Ionization Energy. ...
- Example Problem 2 - Ranking Elements by Ionization Energy. ...
How do you determine ionization energy?
Decide how many electrons the atom contains. This number is the same as Z unless the atom has already lost some electrons. Calculate the ionization energy, in units of electron volts, for a one-electron atom by squaring Z and then multiplying that result by 13.6.
What is ionization energy with example?
Ionization energies measure the tendency of a neutral atom to resist the loss of electrons. It takes a considerable amount of energy, for example, to remove an electron from a neutral fluorine atom to form a positively charged ion. F(g) F+(g) + e- Ho = 1681.0 kJ/mol.
What is the ionization energy of metals?
0:2715:20√ The Ionisation Energy of Metals Explained with Clear Examples ...YouTubeStart of suggested clipEnd of suggested clipSo an ionization is a losing of the electron. Very reactive metals lose electrons readily whileMoreSo an ionization is a losing of the electron. Very reactive metals lose electrons readily while unreactive metals do not lose their electrons readily.
What is 1st ionization energy?
Definition: First Ionization Energy. The first ionization energy is the energy required to remove the most loosely held electron from one mole of neutral gaseous atoms to produce 1 mole of gaseous ions each with a charge of 1+. This is more easily seen in symbol terms.
Which best describes ionization energy?
Which best describes ionization energy? The ionization energy increases because the ratio of the protons to electrons increases.
Is ionization energy only for gases?
While the term ionization energy is largely used only for gas-phase atomic, cationic, or molecular species, there are a number of analogous quantities that consider the amount of energy required to remove an electron from other physical systems.
Does ionization energy increase down a group?
Ionization energy (IE) is the energy required to remove the highest-energy electron from a neutral atom. In general, ionization energy increases across a period and decreases down a group.
Which element is the lowest ionization energy?
CesiumFrom this trend, Cesium is said to have the lowest ionization energy and Fluorine is said to have the highest ionization energy (with the exception of Helium and Neon).
Do metals have high ionization energy?
Metals have high melting points and densities. They are highly malleable and have high ductility. They have low effective nuclear charge, low electronegativity, a large atomic radius, small ionic radius, and low ionization energy.
What are 1st 2nd and 3rd ionization energies?
0:0210:591st 2nd 3rd ionization - YouTubeYouTubeStart of suggested clipEnd of suggested clipThe first ionization energy is removing a single electron. From a neutral atom and converting theMoreThe first ionization energy is removing a single electron. From a neutral atom and converting the atom into a one plus ion second ionization energy is removing a second electron.
What is the 2nd ionization energy?
An element's second ionization energy is the energy required to remove the outermost, or least bound, electron from a 1+ ion of the element. Because positive charge binds electrons more strongly, the second ionization energy of an element is always higher than the first.
What is the highest ionization energy?
heliumThe first ionization energy varies in a predictable way across the periodic table. The ionization energy decreases from top to bottom in groups, and increases from left to right across a period. Thus, helium has the largest first ionization energy, while francium has one of the lowest.
What is ionization energy class 11?
The amount of energy required to liberate the most loosely bound electrons from the outermost shell of an isolated gaseous atom of an element is called ionization energy.
What do you mean by ionization?
ionization, in chemistry and physics, any process by which electrically neutral atoms or molecules are converted to electrically charged atoms or molecules (ions) through gaining or losing electrons.
What is ionization energy class 10?
“It represents the amount of energy required to remove an electron from the valence shell of an isolated gaseous atom (X) in its ground state.” The ionization energy is expressed in units kJ mol - 1 .
What is ionisation energy class 12?
- Hint: Ionization energy is the energy required to remove the electron from the outermost shell of an isolated gaseous atom. The more tightly this outermost electron is bound to the nucleus, more energy is required to remove it.
What is the unit of energy of an electron?
Electronvolt (unit: eV). Electronvolts are a traditional unit of energy particularly in atomic and nuclear physics. Electronvolt is equal to energy gained by a single electron when it is accelerated through 1 volt of electric potential difference. The work done on the charge is given by the charge times the voltage difference, therefore the work W on electron is: W = qV = (1.6 x 10 -19 C) x (1 J/C) = 1.6 x 10-19 J. Since this is very small unit, it is more convenient to use multiples of electronvolts : kilo-electronvolts (keV), mega-electronvolts (MeV), giga-electronvolts (GeV) and so on. Since Albert Einstein showed that mass and energy are equivalent and convertible one into the other, the electronvolt is also a unit of mass. It is common in particle physics, where units of mass and energy are often interchanged, to express mass in units of eV/c 2, where c is the speed of light in vacuum (from E = mc 2 ). For example, it can be said the proton has mass of 938.3 MeV, although strictly speaking it should be 938.3 MeV/c2. For another example, an electron–positron annihilation occurs when a negatively charged electron and a positively charged positron (each with a mass of 0.511 MeV/c 2) collide. When an electron and a positron collide, they annihilate resulting in the complete conversion of their rest mass to pure energy (according to the E=mc 2 formula) in the form of two oppositely directed 0.511 MeV gamma rays (photons).
What is ionization energy?
What is Ionization Energy. Ionization energy, also called ionization potential, is the energy necessary to remove an electron from the neutral atom. Periodic Table
How much ionization energy is needed to remove an electron?
Some of these electrons are more tightly bound in the atom than others. For example, only 7.38 eV is required to remove the outermost electron from a lead atom, while 88,000 eV is required to remove the innermost electron.
How many TeV does a cosmic ray have?
Cosmic ray can have energies of 1 MeV – 1000 TeV.
How much energy is released in a reactor?
The total energy released in a reactor is about 210 MeV per 235 U fission, distributed as shown in the table. In a reactor, the average recoverable energy per fission is about 200 MeV, being the total energy minus the energy of the energy of antineutrinos that are radiated away.
How to find the work of an electron?
The work done on the charge is given by the charge times the voltage difference , therefore the work W on electron is: W = qV = (1.6 x 10-19 C) x (1 J/C) = 1.6 x 10-19 J.
How many EV is needed to remove the outermost electron from a lead atom?
For example, only 7.38 eV is required to remove the outermost electron from a lead atom, while 88,000 eV is required to remove the innermost electron.
What is ionization energy?
Ionization energy: Ionization energy is the minimum energy required to remove an electron from the gaseous atom or ion. In simple words, the electron itself cannot escape out of the orbit.
Can you find every detail of an interactive periodic table?
You can effortlessly find every single detail about the elements from this single Interactive Periodic table.
Can an electron escape an orbit?
In simple words, the electron itself cannot escape out of the orbit. It requires some external energy to be supplied on it in order to escape out of the orbit. And this required energy is known as ionization energy.
What is ionization energy?
Ionization energy, also called ionization potential, is the energy necessary to remove an electron from the neutral atom. X + energy → X+ + e− where X is any atom or molecule capable of being ionized, X+ is that atom or molecule with an electron removed (positive ion), and e− is the removed electron. Periodic Table
How much ionization energy is needed to remove an electron?
Some of these electrons are more tightly bound in the atom than others. For example, only 7.38 eV is required to remove the outermost electron from a lead atom, while 88,000 eV is required to remove the innermost electron. Helps to understand reactivity of elements (especially metals, which lose electrons).
Which metal has the lowest ionization energy?
Ionization energy is lowest for the alkali metals which have a single electron outside a closed shell.
How many eV does sodium need to ionize?
For example, sodium requires only 496 kJ/mol or 5.14 eV/atom to ionize it. On the other hand neon, the noble gas, immediately preceding it in the periodic table, requires 2081 kJ/mol or 21.56 eV/atom.
What is Ionization Energy?
So what is the definition of ionization energy? It is the amount of energy required to remove an electron from a neutral atom, which forms an ion. It is usually measured in kJ/mol, and the measurement is based on an isolated atom in its gaseous phase. Let’s learn how to calculate it, what is meant by first and second ionization energy, and how it trends on the periodic table.
Which element has the highest ionization energy?
In general, (first) ionization energies increase toward the top right corner of the periodic table, with helium having the highest ionization energy. Before we break down the trend into its period and group trends, let’s talk about a major contributing factor to this trend: the octet rule.
Why does ionization decrease when you go down a group?
This is because as you go down a group, electrons are located in successively higher energy levels, farther away from the attraction of the nucleus. Furthermore, down a group, there are more electrons between the outside valence electrons and the nucleus. These middle electrons help “shield” the outer electrons from the attractive forces of the nucleus. Therefore, it is easier to remove an electron from lower in a group.
Why does a group 1 element have very low ionization energies?
Thus, group 1 elements have very low ionization energies. It takes very little energy to remove an electron because the atom can be more stable without it.
How do ions get their charge?
An ion is a positively or negatively charged atom—it gets the charge by having a number of electrons unequal to that of its protons. For example, the sodium ion, also written as Na +, has 11 protons and 10 electrons. There is one more proton than there are electrons, making the ion positively charged. The number of protons for any atom or ion is always constant (the number of protons determines the atomic number).
What is the energy of an electron?
E is energy of the electron (or the amount of energy it takes to remove the electron, ionization energy)
Which group of gases has the highest ionization potential?
As you can see on the graph, the noble gases have the highest ionization energies, and the alkali metals have the lowest ionization energies. Between groups 1 and 18, ionization potentials generally increase across a period.
What is ionization energy?
Ionization energy is the minimum energy required to remove an electron from an atom or ion in the gas phase. The most common units of ionization energy are kilojoules per mole (kJ/M) or electron volts (eV). Ionization energy exhibits periodicity on the periodic table. The general trend is for ionization energy to increase moving from left ...
What is the trend for ionization energy to decrease moving from top to bottom down a periodic table group?
The general trend is for ionization energy to decrease moving from top to bottom down a periodic table group. Moving down a group, a valence shell is added. The outermost electrons are further from the positive-charged nucleus, so they are easier to remove.
Why does ionization decrease as electrons move down the group?
This is because the principal quantum number of the outermost electron increases moving down a group. There are more protons in atoms moving down a group (greater positive charge), yet the effect is to pull in the electron shells, making them smaller and screening outer electrons from the attractive force of the nucleus. More electron shells are added moving down a group, so the outermost electron becomes increasingly distance from the nucleus.
What are the exceptions to the ionization energy trend?
Exceptions to the Ionization Energy Trend. If you look at a chart of first ionization energies, two exceptions to the trend are readily apparent. The first ionization energy of boron is less than that of beryllium and the first ionization energy of oxygen is less than that of nitrogen.
Why is ionization energy important?
Ionization energy is important because it can be used to help predict the strength of chemical bonds. Also Known As: ionization potential, IE, IP, ΔH°. Units: Ionization energy is reported in units of kilojoule per mole (kJ/mol) or electron volts (eV).
What happens to ionization energy when moving from left to right?
Moving left to right across a period, atomic radius decreases, so electrons are more attracted to the (closer) nucleus.
What is the trend of ionization?
Ionization, together with atomic and ionic radius, electronegativity, electron affinity, and metallicity, follows a trend on the periodic table of elements. Ionization energy generally increases moving from left to right across an element period ( row). This is because the atomic radius generally decreases moving across a period, ...
What is the unity of ionization energy?
The unity for ionization energy is eV.
How many elements are in chemistry?
This list contains the 118 elements of chemistry.
What is ionization energy?
Explanation: Ionization energy is the energy needed to remove one electron from an atom in the gaseous state. This electron would be a valence electron, or an electron in the outermost energy level/shell, because they're the easiest to remove. Ionization energy depends mainly on the strength of the attraction between the negative electron and ...
Which element has a higher first ionization energy?
Magnesium is found to have a higher first ionization energy value than aluminum, how would you explain this exception to the general trend in terms of electron arrangements and attraction/repulsion?
What happens when you move down a group in the periodic table?
When we move down a group in the periodic table, more energy levels are added, and so valence electrons would become further and further away from the positive nucleus. This causes the attraction between valence electrons and the nucleus to decrease, something known as the shielding effect. The less attraction between the electrons and ...
