Full Answer
What are the 6 types of kinetic energy?
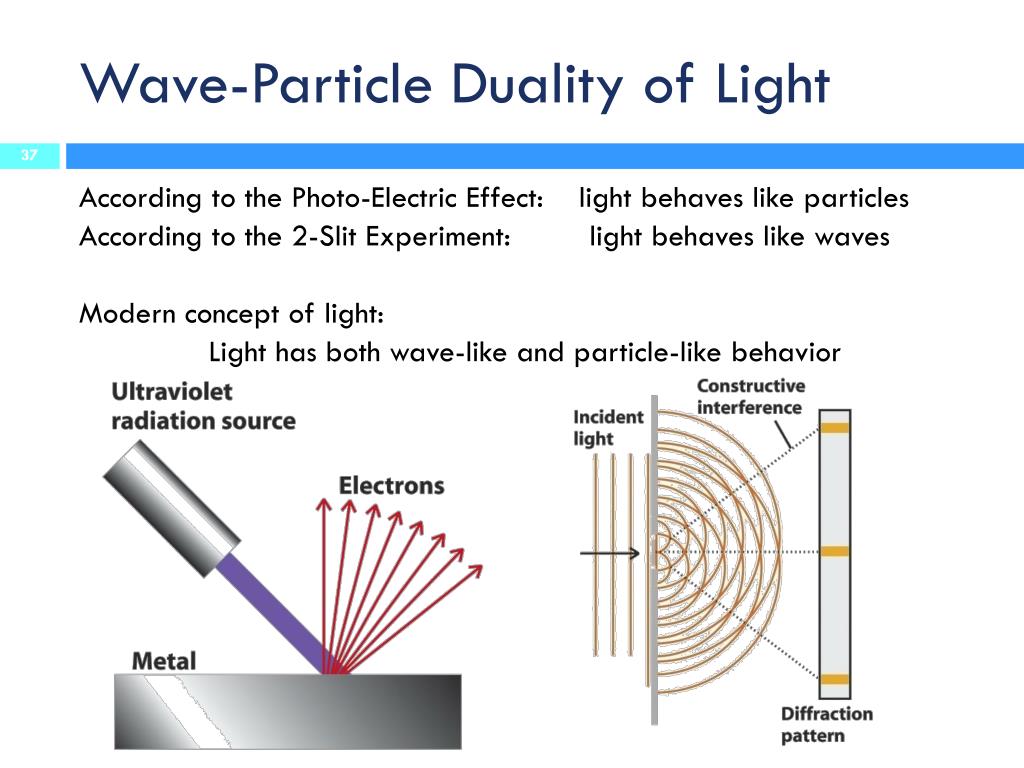
Types of kinetic energy include radiant energy, thermal energy, sound energy, electrical energy ... As we know that kinetic energy is the energy of motion. Radiant energy is always in motion by travelling through space or medium. Hence, it is a kind of kinetic energy. ...
What are 3 examples of kinetic energy?
Kinetic Energy Examples
- Radiant Energy Examples. Radiant energy is a type of kinetic energy, referring to energy that travels by waves or particles. ...
- Thermal Energy. Thermal energy is similar to radiant energy in that both can be experienced in the form of heat or warmth.
- Sound Energy. The human experience of sound is caused by vibrations. ...
- Electrical Energy. ...
- Mechanical Energy. ...
What is the potential energy of an electron?
for a medium sized nucleus (its roughly between 1,75 fm for Hydrogen to 15 fm for Uranium, and the numerical value of ℏ = 1, 054.10 − 34 J s it turns out that the energy of the electron (converting from momentum using the relativistic basic equation E 2 = p 2 c 2 + m 2 c 4) is about 40 MeV.
What are some real life examples of kinetic energy?
What is a real life example of kinetic energy?
- Moving Car. Moving cars possess some amount of kinetic energy.
- Bullet From a Gun. A bullet fired from a gun has very high kinetic energy, and, so, it can easily penetrate any object.
- Flying Airplane.
- Walking & Running.
- Cycling.
- Rollercoasters.
- Cricket Ball.
- Skateboarding.

What is the kinetic energy of the emitted electron?
The maximum energy of an emitted electron is equal to the energy of a photon for frequency f (i.e., E = hf ), minus the energy required to eject an electron from the metal's surface (the so-called work function).
Do the emitted electron have same kinetic energy?
In process of photoelectric emission, all emitted electrons do not have same kinetic energy.
What is the maximum kinetic energy of emitted electrons?
In a photoelectric effect experiment, the threshold wavelength of incident light is 260 nm and E (in eV) = 1237/λ (nm). Find the maximum kinetic energy of emitted electrons. Therefore, the maximum kinetic energy of emitted electrons in the photoelectric effect is 1.5 eV.
What is the energy of ejected electron?
The kinetic energy of an ejected electron equals the photon energy minus the binding energy of the electron in the specific material. An individual photon can give all of its energy to an electron. The photon's energy is partly used to break the electron away from the material.
Why does kinetic energy of emitted electrons vary?
I think its because when the light hits the surface, photons of energy are given to the electrons, and the energy of the photons vary, so not every electron would get hit by photons of the same energy levels, giving them a different amount of kinetic energy.
Do the emitted photoelectrons have the same kinetic energy Why?
Yes, all the emitted photoelectrons have the same kinetic energy as the kinetic energy of the emitted photoelectrons depends upon the frequency of the incident radiation for a given photosensitive surface.
Why is the maximum kinetic energy of emitted electron does not change?
Photons are absorbed instantly so electron emitted instantly. Increasing intensity means more photons (per second, per m2) so results in more electrons emitted (per second) but no change to max KE. Increasing frequency means more energy per photon so higher max KE of electrons but same rate of electron emission.
Does kinetic energy of emitted electrons depend upon?
Solution : Kinetic energy of emitted electron depends on the frequency of incident radiation.
How do you find the maximum kinetic energy of a photoelectron?
This value is the maximum possible kinetic energy of the photoelectron. The equation, which Einstein determined, says (electron's maximum kinetic energy) = (energy of the incident light energy packet) minus (the work function). For the example, the electron's maximum kinetic energy is: 2.99 eV - 2.75 eV = 0.24 eV.
How do you measure kinetic energy of an electron?
Therefore, we calculate the kinetic energy using the equation E(photon) = E(threshold) + KE. Then, we can use the equation for kinetic energy (KE = 1/2 mv2) and substituting in the mass of an electron (9.11 x 10-31 kg), we can calculate the velocity for the single electron.
How do you find the ejected electron KE?
Re: How to find the kinetic energy of ejected electrons? Kinetic energy is calculated through the formula KE= 0.5 m*v2, where m is the mass, in this case the ejected electron mass, and v is the velocity which is given in the question.
How do you find the kinetic energy of a photoelectric effect?
The maximum kinetic energy of a photoelectron is given by 𝐸 = ℎ 𝑓 − 𝑊 , m a x where ℎ is the Planck constant, 𝑓 is the frequency of the incident photon, and 𝑊 is the work function of the metal surface.
Do you expect all the ejected electrons to have the same kinetic energy?
Answer and Explanation: No, it is not necessary that all the ejected electrons will have the same energy. Because the kinetic energy of the electron is the excess energy of the binding energy of the electron which is also known as work function. So some electrons receive more and some receive less.
Does kinetic energy of emitted electrons depend on the intensity of incident radiation?
KE does not depend on the intensity fo incident radiation. Q. When light of sufficiently high frequency is incident on a metallic surface, electrons are emitted from the metallic surface. This phenomenon is called photoelectric emission.
Do all photoelectrons emitted from a metal surface possess the same energy?
All photo electrons are not emitted with the same energy as the incident photons.
Do all photoelectrons have the same energy?
All the photoelectrons are not emitted with same energy.
What is the K.E. of photoelectron?
K.E. of photoelectron = (KE of incident photon) - (Work Function of Metal).
How often does the work function have to be paid off?
Likewise, the work function has to be paid off only once, for the electron to surface. It has got nothing to do with the incident energy of the photon.
Is incident photon 1 more than photoelectron 1?
BUT, KE of incident Photon 1 is MORE than the KE of photoelectron 1 (by first equation.)
Maximum possible kinetic energy of the emitted electrons
If molybdenum is irradiated with 194 nm light, what is the maximum possible kinetic energy of the emitted electrons? The calculated minimum work is 7.22x10^-19J
Re: Maximum possible kinetic energy of the emitted electrons
If molybdenum is irradiated with 194 nm light, what is the maximum possible kinetic energy of the emitted electrons? The calculated minimum work is 7.22x10^-19J
Re: Maximum possible kinetic energy of the emitted electrons
I tried this equation on my own, and then see that the same one was used here. However, when I calculated the answer using these exact numbers, I kept getting a value that was equal (but negative) to the minimum Ek (which was given). Is this an issue with how I am using may calculator? Does anyone know why I am getting this number in particular?
Re: Maximum possible kinetic energy of the emitted electrons
Sophia Stewart 2G wrote: I tried this equation on my own, and then see that the same one was used here. However, when I calculated the answer using these exact numbers, I kept getting a value that was equal (but negative) to the minimum Ek (which was given).
Re: Maximum possible kinetic energy of the emitted electrons
Sophia Stewart 2G wrote: I tried this equation on my own, and then see that the same one was used here. However, when I calculated the answer using these exact numbers, I kept getting a value that was equal (but negative) to the minimum Ek (which was given).
Re: Maximum possible kinetic energy of the emitted electrons
Sophia Stewart 2G wrote: I tried this equation on my own, and then see that the same one was used here. However, when I calculated the answer using these exact numbers, I kept getting a value that was equal (but negative) to the minimum Ek (which was given).
Re: Maximum possible kinetic energy of the emitted electrons
Yeah, I was thinking it had to be something like that. thanks for your help!
