What are the hazards of calcium hydroxide?
What Are the Dangers of Calcium Hydroxide?
- Ingestion. Accidental ingestion of calcium hydroxide can cause severe throat pain, a burning sensation in the mouth, abdominal pain, vomiting, bloody stool or vomit, rapidly falling blood pressure and collapse, ...
- External Exposure. ...
- Inhalation. ...
- Chronic Exposure. ...
How do you calculate the Ksp for Ca(OH)2?
The equation for the Ksp of Ca(OH)2 is the concentration [Ca2+] times the concentration [OH-] taken to the second power, since the OH- has a coefficient of 2 in the balanced equation. Plug the concentrations of each of the products into the equation to calculate the value of Ksp.
How do you calculate KSP from molar solubility?
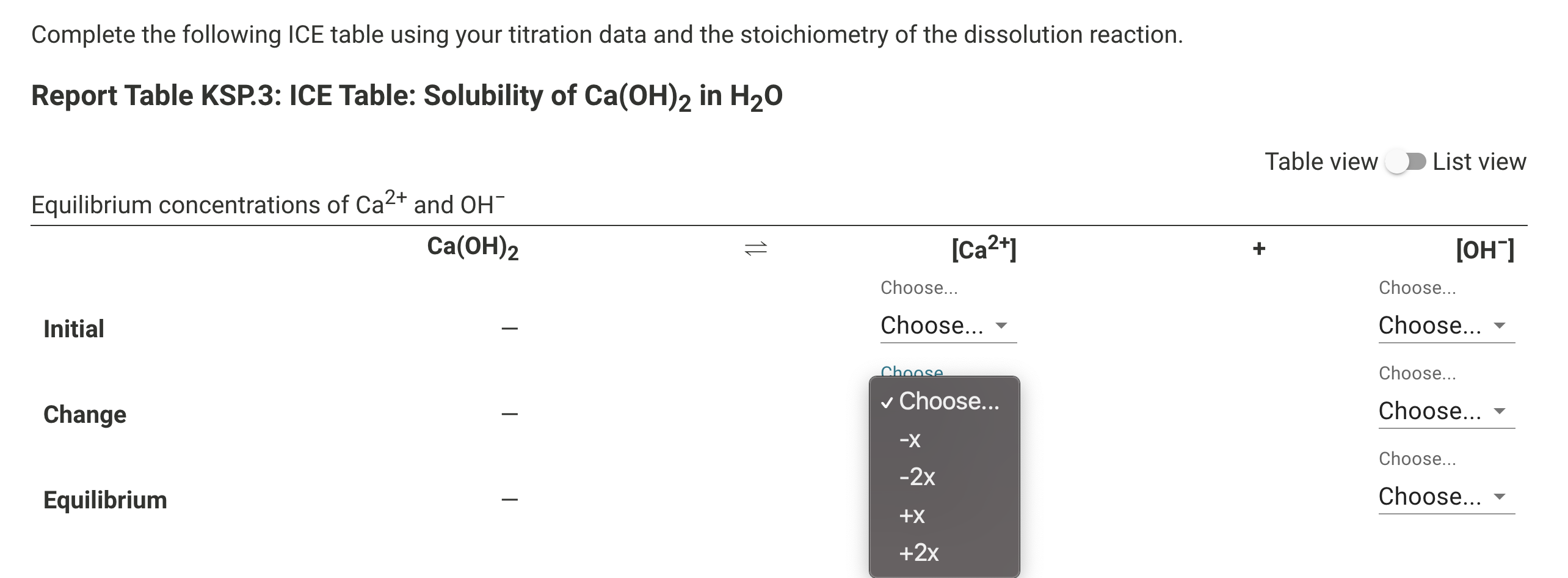
- Set up an ICE problem (Initial, Change, Equilibrium) in order to use the Ksp value to calculate the concentration of each of the ions.
- The concentration of the ions leads to the molar solubility of the compound.
- Use the molar mass to convert from molar solubility to solubility.
What is the chemical equation for calcium hydroxide?
What is the chemical formula of calcium hydroxide? Answer: Calcium hydroxide is formed by one (mathrm{Ca}^{2+}) cation and two (O H^{-}) nions, which implies that its chemical formula will be (mathrm{Ca}(mathrm{OH})_{2})

How do you calculate Ksp of calcium hydroxide?
The apparent equilibrium constant, Ksp, can be calculated from the molar solubility of calcium hydroxide: Ksp = [Ca2+][OH-]2. The for the dissolution of calcium hydroxide at each temperature is calculated from the formula = -RT ln(Kc).
What is the solubility of calcium hydroxide Ksp?
The solubility product (Ksp) of Ca(OH)2 at 25∘C is 4.42×10−5.
What is the Ksp for calcium hydroxide at 25 degrees Celsius?
Calcium hydroxide has Ksp = 5.5 × 10−5 at 25 degrees C and a molar mass of 74.10 g/mol. How many grams of calcium hydroxide are needed to create 365 mL of a saturated solution of calcium hydroxide at 25 degrees C?
Is Ca OH 2 Ksp?
The solubility product (Ksp) of Ca(OH)2 at 25∘C is 4. 42×10−5. A 500 ml of saturated solution of Ca(OH)2 is mixed with equal volume of 0.4M NaOH.
How do you calculate Ksp from solubility?
For each compound, the molar solubility is given. Calculate its Ksp....Here is a skeleton outline of the process: Write the chemical equation for the substance dissolving and dissociating. Write the Ksp expression. Insert the concentration of each ion and multiply out.
What is the Ksp of Ca OH 2 at room temperature?
The tabulated Ksp for Ca(OH)2 is 6.5 x10-6 at 25°C. 2. In general, the solubility of most salts increases as the temperature increases. Based on your results and the comparison to the pKsp values given above, how does temperature affect solubility of Ca(OH)2?
How do you calculate average Ksp?
Ksp = [Ca2+][OH-]2 Remember that the calcium ion concentration is half the hydroxide ion concentration determined by the titration.
What is a Ksp value?
The solubility product constant, Ksp, is the equilibrium constant for a solid substance dissolving in an aqueous solution. It represents the level at which a solute dissolves in solution. The more soluble a substance is, the higher the Ksp value it has.
How do you calculate Ksp from concentration?
2:454:36Calculate Ksp using one ion concentration - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo all you need to do now to find KSP is substitute in your known concentration. So you know thatMoreSo all you need to do now to find KSP is substitute in your known concentration. So you know that your sodium concentration is three point one zero. Times 10 to the negative sixth.
What is the Ksp of NaOH?
SOLVED: Calculate Ksp for NaOH at 25 °C. Na+(aq) OH-(aq) NaOH(s) ΔG°f (kJ/mol) -261.9 -157.3 -379.7 answer: 1.09E7.
What is the molar solubility of Ca OH 2?
. Calculate the molar solubility. 3) The solubility of Ca(OH)2 is found to be 0.233 g/L.
What is the Ksp of CaSO4?
Ksp of CaSO4 is 4 × 10^-12.
What is the Ksp for CaCO3?
Calcium carbonate, CaCO3 has a Ksp value of 1.4 × 10^-8 .
What is the Ksp of CaCl2?
1 g / 100 m L . Thus, the Ksp K s p value for CaCl2 C a C l 2 is 21. 33×108g/L.
What is the Ksp of CaSO4?
Ksp of CaSO4 is 4 × 10^-12.
What is the Ksp of NaOH?
SOLVED: Calculate Ksp for NaOH at 25 °C. Na+(aq) OH-(aq) NaOH(s) ΔG°f (kJ/mol) -261.9 -157.3 -379.7 answer: 1.09E7.
1. Justify Calcium Hydroxide, Whether it is Acidic or Basic?
Calcium hydroxide is also called slaked lime (having the chemical formula Ca(OH)2). It is a hydroxide ion source when dissolved in aqueous solution...
2. How is Calcium Hydroxide Applied in Dental and Medical Application?
Calcium hydroxide is a white powdered compound that does not contain any characteristic odor. Also, there are various dental and medical applicatio...
3. How is Calcium Hydroxide Prepared?
Calcium hydroxide is also known as slaked lime in general. It is in white when it is in a solid-state and colorless when it is in crystallized form...
4. Provide Some Asian Uses of Calcium Hydroxide.
Calcium hydroxide is formed by mixing water and calcium oxide also known as quicklime. It is white when it is in a solid-state and colorless when i...
5. What are the general uses of calcium hydroxide?
Calcium hydroxide, also known as slaked lime in general, is represented as Ca(OH)2 and it is an inorganic compound. When it is in the solid-state,...
What is the KSP of calcium hydroxide?
The solubility product (Ksp) of the calcium hydroxide can be given as 5.5 * 10-6.
How is calcium hydroxide formed?
Ans: Calcium hydroxide compound is formed by the action of water on calcium oxide, which is also called slaked lime (Ca (OH)2). A lesser proportion of it dissolves when it is combined with water, forming a solution called limewater, the remainder remaining in a suspension known as lime milk.
How are calcium hydroxide molecules held together?
The calcium hydroxide molecules are held together by ionic bonds between the two hydroxide ions (OH–) and calcium ions (Ca2+). Unprotected exposure to this compound may prove dangerous to humans by leading to skin irritation and chemical burns. Exposure to the concentrated Ca (OH)2 can lead to damage to the lungs and even blindness.
What metals does calcium hydroxide dissolve?
The saturated calcium hydroxide solution in water also reacts with and dissolves the metals such as aluminum.
What is the chemical reaction between calcium chloride and sodium hydroxide dissolved in water?
Also, the chemical reaction between the calcium chloride and sodium hydroxide dissolved in water (aqueous CaCl2) yields this compound. The structural representation of a Ca (OH)2 molecule can be illustrated below.
Is calcium hydroxide a base compound?
It is a hydroxide ion source when dissolved in aqueous solutions. Thus, this is a base compound. Due to the electrolyte dissociation, this compound liberates the OH- ions.
What is the name of the solution of calcium hydroxide?
Aqueous solutions of calcium hydroxide are called limewater and are medium-strength bases, which reacts with acids and can attack some metals such as aluminium (amphoteric hydroxide dissolving at high pH), while protecting other metals, such as iron and steel, from corrosion by passivation of their surface.
What is the chemical formula for calcium hydroxide?
Chemical compound. Calcium hydroxide (traditionally called slaked lime) is an inorganic compound with the chemical formula Ca ( OH) 2. It is a colorless crystal or white powder and is produced when quicklime ( calcium oxide) is mixed or slaked with water.
Why does calcium hydroxide have a retrograde solubility?
This counter-intuitive temperature dependence of the solubility is referred to as "retrograde" or "inverse" solubility. The variably hydrated phases of calcium sulfate ( gypsum, bassanite and anhydrite) also exhibit a retrograde solubility for the same reason because their dissolution reactions are exothermic.
How is calcium hydroxide produced?
Calcium hydroxide is produced commercially by treating lime with water:
What is the temperature of water in equilibrium with calcium hydroxide?
When heated to 512 °C, the partial pressure of water in equilibrium with calcium hydroxide reaches 101 kPa (normal atmospheric pressure), which decomposes calcium hydroxide into calcium oxide and water:
What is a Ca supplement?
In fortifying (Ca supplement) fruit drinks, such as orange juice, and infant formula. As a digestive aid (called Choona, used in India in paan, a mixture of areca nuts, calcium hydroxide and a variety of seeds wrapped in betel leaves) As a substitute for baking soda in making papadam.
Is calcium hydroxide soluble in water?
Calcium hydroxide is poorly soluble in water with a retrograde solubility increasing from 0.66 g/L at 100 °C to 1.89 g/L at 0 °C. With a solubility product Ksp of 5.5 × 10 −6 at T = ?. its dissociation in water is large enough that its solutions are basic according to the following dissolution reaction:
What is KSP in chemistry?
Ksp for Calcium Hydroxide. Introduction: Calcium hydroxide is a soft white caustic powder used in making mortar, cements, calcium salts, paints, and petrochemicals. It is also used in saltwater aquaria to make up kalkwasser/limewater solutions for reef tanks, and is used as a pH regulating agent. Notice that calcium hydroxide is divalent ...
What is a saturated solution of calcium hydroxide?
A calcium hydroxide solution is also referred to as limewater. A saturated solution of calcium hydroxide has the solid in equilibrium with its ions as shown below:
What is the indicator used to measure the volume of saturated calcium hydroxide solution?
The indicator used will be phenolphthalein. From the volume of saturated calcium hydroxide used, you will be able to determine its concentration and thus its Ksp value.
What happens when calcium hydroxide is saturated?
A saturated solution of calcium hydroxide must be made fresh on the day it is to be used as any carbon dioxide that enters the solution will cause it to react to form a calcium carbonate precipitate (chalk), as shown in the first two pictures below:
How to measure the concentration of hydroxide ions formed when Ca(OH)2 dissolves?
The concentration of hydroxide ions formed when Ca(OH)2 dissolves can be measured using the titration technique. An acid-base titration is a process in which a measured volume of an acid or base is added to a reaction mixture until the acid-base indicator changes color. In the procedure used in this lab, a dilute solution of HCl is titrated with a saturated solution of Ca(OH)2 to the endpoint of phenolphthalein.
How is calcium hydroxide made?
Calcium hydroxide is manufactured industrially by adding water to calcium oxide (quicklime) in a strongly exothermic reaction:
How to fill a buret with HCl?
Rinse a second buret a couple of times with the HCl solution. Then fill the buret to above its zero mark. Open the stopcock briefly to allow any air bubbles to pass through. (Again, why is this important?) Record the initial volume of HCl in the buret to the nearest 0.05 mL.