
Full Answer
What is KSP in chemistry?
Background: Ksp : K is the solubility product constantfor an ionic compound. Recall in Chapter 4 Section 5, a series of rules were given to determine whether an ionic compound was soluble or insoluble in water. The solubility product constant is the numerical value that explains the entries in the table. The larger the K sp
How do you calculate K sp for KHT?
2 O (l) + NaT-(aq) Then by using an ICE chart along with the solubility of KHT (which equals the concentration of HT-), K sp can be calculated for KHT at the temperature for the experiment. KHT (s) K+(aq) + HT-(aq) K sp Titration of a Saturated Potassium Hydrogen Tartrate (KHT) Solution Against Standardized NaOH
How do you record volume of saturated KHT in a flask?
Using the 25-mL volumetric pipet, transfer 25-ml of the saturated KHT solution into a 125-mL Erlenmeyer Flask and record on the data sheet as Volume of HT-to the 0.01 mL. 10. Repeat 1 more times into an additional 125-mL flasks.
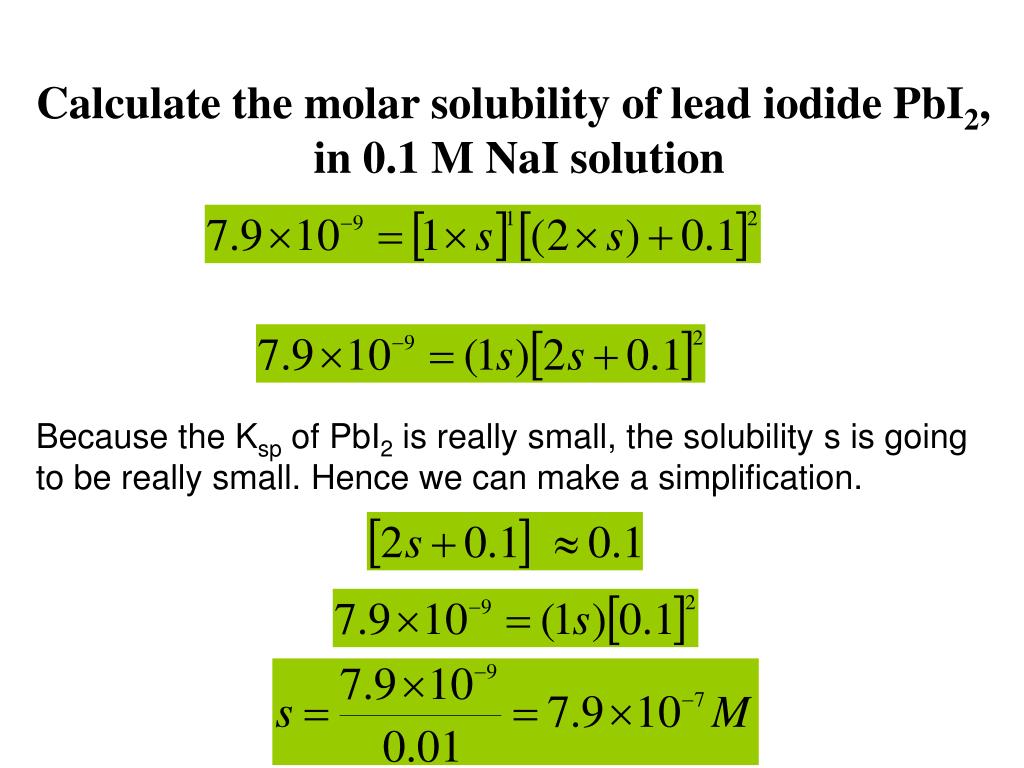
How do you find KSP from KHT?
4:067:00Calculating the Solubility Product Constant of KHTartrate - YouTubeYouTubeStart of suggested clipEnd of suggested clipWe square the molarity of the saturated potassium hydrogen tartrate and that gives us the solubilityMoreWe square the molarity of the saturated potassium hydrogen tartrate and that gives us the solubility. Product constant at that temperature. So you saturate the solution you collect the filtrate.
What does the KSP tell you about the solubility of KHT?
Ksp is the product of dissolved ions saturation as a fraction of their coefficients. Thus, the calculated molarity of NaOH is used to compute the molar solubility of KHT, which is the quantity of KHT moles that are liquefied in every liter before saturation level.
How KSP is calculated?
Next we write out the expression for Ksp , then "plug in" the concentrations to obtain the value for Ksp. Let's do an example: The solubility of Ag2CrO4 in water is 1.31 x 10-4 moles/L. Calculate the value of Ksp . Using mole ratios, the [Ag+] will go up by (2 x 1.31 x 10-4 moles/L) = 2.62 x 10-4 moles/L.
What is the KSP of KHC4H4O6?
The literature Ksp value for KHC4H4O6 is 3.8 x 10-4 at 291.15K.
How do you calculate Ksp from solubility?
0:492:55How to Calculate Solubility from Solubility Product - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo my ksp will be a g plus concentration which is x b r minus concentration which is also x soMoreSo my ksp will be a g plus concentration which is x b r minus concentration which is also x so therefore this would be an x squared.
Is KHT more soluble in water or KCl?
Essentially, the solubility of KHT decreases as the concentration of KCl increases. Results of the experiment (see table 1) indicate that the solubility values of KHT in water, 0.05M KCl, and 0.1M KCl are 8.952 × 10-4 moles/L, 2.67 × 10-4 moles/L, and 1.0282 × 10-4 moles/L respectively.
What does Ksp stand for?
Solubility Product ConstantSolubility Product Constant, Ksp.
Is Ksp the same as molar solubility?
We can calculate the molar solubility using Ksp, but we have to know the ions produced by the dissociation during the dissolution of the substance in the solution. Here, x is the molar solubility. Therefore, if we know the Ksp of the reaction, we can calculate the x, molar solubility of the reaction.
How do you find Ksp units?
Actually, it doesn't have a unit! The K s p value does not have any units because the molar concentrations of the reactants and products are different for each equation.
What is KHT in chemistry?
Molecular Formula. C4H6KO6+ Synonyms. Potassium hydrogen tartrate.
Is KHT an acid or base?
Potassium bitartrate, also known as potassium hydrogen tartrate, with formula KC4H5O6, is a byproduct of winemaking. In cooking, it is known as cream of tartar. It is processed from the potassium acid salt of tartaric acid (a carboxylic acid)....Potassium bitartrate.NamesLD50 (median dose)22 g/kg (oral, rat)27 more rows
What is the effect of increasing the concentration of KCl on the water solubility of KHT?
The solubility of the KHT in these various solutions is determined by titration with standardized NaOH solution. The results show that the solubility of KHT is unaffected by changing the glucose concentration, decreases with increasing KCl concentration, and increases with increasing NaCl and MgSO4 concentration.
What does a higher molar solubility mean?
0:0141:52Ksp - Molar Solubility, Ice Tables, & Common Ion Effect - YouTubeYouTubeStart of suggested clipEnd of suggested clipIn this video we're going to talk about how to calculate the ksb value given the molar solubility.MoreIn this video we're going to talk about how to calculate the ksb value given the molar solubility.
How does temperature affect the solubility of KHT?
2 Answers. Usually, increasing the temperature increases the solubility of solids and liquids.
How do you find molar solubility from KSP?
1:185:48How to Solve for Ksp Given Molar Solubility (Shortcut and ... - YouTubeYouTubeStart of suggested clipEnd of suggested clipThen we can substitute the variables into the expressions. So KSP will just equal x squared. X isMoreThen we can substitute the variables into the expressions. So KSP will just equal x squared. X is equal to the molar solubility.
Homework Statement
Determine [K+] and [HT-] in this solution. If the temperature is Tp, a trace of solid is present and reaction is at equilibrium. Determine Ksp at this temperature.
The Attempt at a Solution
I originally thought to set up an ICE table to find the concentrations of K+ and HT- at equilibrium. With the concentrations, I figured I could then multiply them to get my Ksp value. I realized, however, I do not have a Kc value to use to solve for x. Is there any other way to find Ksp that I'm just missing?
Answers and Replies
No need for ICE table, all you need to calculate concentrations is a molar mass of KHT.
What is potassium hydrogen tartrate?
More... Potassium hydrogen tartrate is a L-alpha-D- Hepp - (1->7)-L-alpha-D- Hepp - (1->3)-L-alpha-D- Hepp - (1->5)- alpha-Kdo. Potassium bitartate, also referred to as potassium acid tartrate or cream of tartar, is the potassium acid salt of l- ( + )-tartaric acid. It is obtained as a byproduct of wine manufacture during the fermentation process. ...
How does potassium bitartrate work?
Potassium bitartrate is a carbon dioxide -releasing laxative that works by forming carbon dioxide gas, which creates a mechanical distension against the intestinal wall and induces bowel contractions. Rectal suppositories of carbon dioxide -releasing type of laxative were demonstrated to be useful and safe in the treatment of patients at risk for electrolyte disorders such as the elderly or patients with renal or cardiovascular disorders.
What is the name of the potassium acid salt?
Potassium hydrogen tartrate is a L-alpha-D- Hepp - (1->7)-L-alpha-D- Hepp - (1->3)-L-alpha-D- Hepp - (1->5)- alpha-Kdo. Potassium bitartate, also referred to as potassium acid tartrate or cream of tartar, is the potassium acid salt of l- ( + )-tartaric acid. It is obtained as a byproduct of wine manufacture during the fermentation process.
