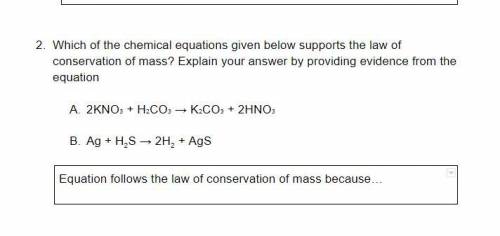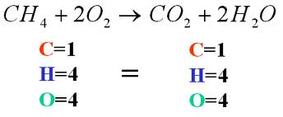
How can we prove the law of Conservation of mass?
When balancing chemical equations, the law of conservation of mass is also demonstrated because the total number of atoms that goes into the reaction must be produced. So if 14 atoms are on the reactant side, then 14 atoms must be on the product side. Equations often require balancing to correctly demonstrate the law of conservation of mass.
What is the equation for the law of conservation?
What is the law of conservation of energy equation? The equation expressing conservation of energy is: KEi+PEi=KEf+PEf. If you know the potential energy for only some of the forces, then the conservation of energy law in its most general form must be used: KEi+PEi+Wnc+OEi=KEf+PEf+OEf, where OE stands for all other energies.
What is equation follows the law of Conservation of mass?
There are several ways to consider the law of conservation of mass examples. In a simple combination reaction where two substances are chemically combined, we will see conservation as follows: 300g A + 100g B --> 400g AB.
How to solve law of Conservation of mass problems?
How to Solve Law of Conservation of Mass Problems ... Balancing a reaction equation is one way of solving a conservation of mass problem. To do this, you recognize that both sides of the equation contain the same number of atoms of each element involved in the reaction.
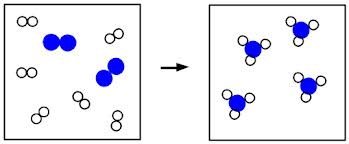
How do you calculate law of conservation of mass?
Since atoms are not lost or made in a chemical reaction, the total mass of the products is equal to the total mass of the reactants . The sum of the relative formula masses of the reactants is equal to the sum of the relative formula masses of the products.
What is law of conservation of mass explain?
The law of conservation of mass states that mass in an isolated system is neither created nor destroyed by chemical reactions or physical transformations. According to the law of conservation of mass, the mass of the products in a chemical reaction must equal the mass of the reactants.
What are 3 laws of conservation of mass?
What is the Law of Conservation of Mass? “The mass in an isolated system can neither be created nor be destroyed but can be transformed from one form to another”. According to the law of conservation of mass, the mass of the reactants must be equal to the mass of the products for a low energy thermodynamic process.
What is law of conservation of mass with example?
The law of conservation of mass states that matter cannot be created or destroyed in a chemical reaction. For example, when wood burns, the mass of the soot, ashes, and gases equals the original mass of the charcoal and the oxygen when it first reacted.
Why is there no change in mass during chemical reactions?
During a chemical reaction, atoms are neither created nor destroyed. The atoms of the reactants are just rearranged to form products. Hence, there...
Verify law of conservation of mass with an experiment
According to the law of conservation of mass, during any physical or chemical change, the matter is neither created nor destroyed. However, it may...
If energy is neither created nor destroyed, what is the ultimate source of energy?
The ultimate source of energy in our present universe is the Big Bang. All the energy was created at the beginning of time and as the universe grew...
1. State the Law of Conservation of Mass and Energy.
The mass and energy are inconvertible; however, their total during any physical or chemical change remains conserved.In a nuclear reaction, the mas...
2. If 4.2 g of KClO3 is Heated to Produce 1.92 g of O and 2.96 g of KCl as Residue. Show that this R...
KClO3 → KCl + \[\frac {3}{2}\] O2 Sum of masses of products1.92 + 2.96 = 4.88 gThe difference between the total mass of reactants and products and...
3. How is the law of conservation chapter in physics used in our daily life?
Physics gets highly involved in our daily routine right from you wake up in the morning until you go back to bed. Examples of the law of conservati...
4. Does the Law of Conservation of Energy apply to machines?
We are, with or without our knowledge actually using the law of conservation of mass in our everyday life simple activities such as walking, playin...
5. What is the law of conservation of mass state?
The law of conservation of mass states that in a chemical reaction between any object the mass that is produced by them is neither created nor dest...
6. Why is this Law of Conservation of the mass Chapter so important?
This chapter helps us to understand the laws and rules that govern the entire physical world around us which we are not aware of most of the time....
7. Why does the law of conservation of mass only apply to chemical changes?
The law of conservation of mass states that mass in any particular isolated system can neither be created nor destroyed by chemical reactions or an...
What is the Law of Conservation of Mass?
The Law of conservation of mass was studied by a French Chemist named Antoine Lavoisier in 1789.
What is the name of the solution in a Landolt's tube?
One tube contains a solution of sodium chloride and another contains a silver nitrate solution; the tube containing these two solutions is called Landolt’s tube.
How to melt ice into water?
Take a piece of ice (ice is solid water) and place it in a conical flask . This flask is properly corked and weighted and it is now heated gently to melt the ice into water .
What happens when 100 g of HgO is heated?
When 100 g of HgO is heated, it decomposes into two compounds viz: one mole of Hg and a half mole of O2. The reaction occurs in the following manner:
Why does the weight of a flask remain the same?
(solid) (Liquid) The flask is weighed again, we notice that the weight of the flask remains the same because the mass of ice does not change after it undergoes a physical change. When Matter Undergoes a Chemical Change .
How many grams are in 2 moles of H2O?
So, we get the mass of 2 moles of H2O as 36 g.
What happens to the mass of wood after burning?
If we weigh the piece of wood and after burning it, ashes, water vapors, and CO2 are heated, there will not be any change in the mass before and after the reaction.
How are chemical reactions expressed?
Chemical reactions are usually expressed in terms of chemical equations where reactants change to form products. Chemical equations are the shorthand representation of a chemical reaction in terms of atoms and molecules. In a chemical equation, the number of atoms reacting is always equal to the number of atoms formed. This means the right and left sides of the arrow in a chemical equation should have an equal number of atoms resulting in a balanced chemical equation. A balanced chemical equation is based on the Law of conservation of mass proposed by Antoine Lavoisier. Through this article, we learned the statement, concept and examples that govern the Law of conservation of mass. We also learned the Law of conservation of mass energy, Law of conservation of momentum and its formula.
How does conservation of mass help us in chemical reactions?
The Law of Conservation of mass allows us to represent a chemical reaction as a balanced equation, in which the number of moles of any element on the reactant side is the same as that on the product side. This Law also helps us to determine the masses of gaseous reactants and products. With the sum of the masses of the solid or liquid reactants and products, any remaining mass can be assigned to gas.
What is the total mass of the reactants?
In this case, the total mass of the reactants = is the total mass of the products. Also, the number of atoms of oxygen and hydrogen in the reactants side and the products side are equal.
What is the law of conservation of mass?
The Law of conservation of mass states that, in a chemical reaction or physical transformation, the mass of the participating species is conserved, i.e., it can neither be created nor destroyed within an isolated system . In simple words, the mass of the products formed in a chemical reaction will always be equal to the mass of the reactants.
When a candle melts, what is the mass of the soot and gases?
For example, when a candle melts, the mass of the soot and gases equals the original mass of the wax and the oxygen when it first reacted. Hence, the mass of the substances is equal before and after a chemical reaction. i.e. the mass of the products formed is equal to the mass of the reacting species.
What is Newton's third law?
According to Newton’s Third Law, when a force is applied by object A on object B, then object B exerts back an equal and opposite force on object A. This idea was the underlying principle behind the Law of conservation of momentum.
Is the total mass of reactants and products equal?
In this case, the total mass of reactants and products also are equal. The number of atoms at the beginning and the end is equal as well.
Why is the law of conservation of mass important?
The law of conservation of mass was crucial to the progression of chemistry, as it helped scientists understand that substances did not disappear as result of a reaction (as they may appear to do); rather, they transform into another substance of equal mass. History credits multiple scientists with discovering the law of conservation of mass.
Which law states that a balanced chemical equation has the same mass of reactants and products?
According to the Law of Conservation of Mass, a balanced chemical equation has the same mass of reactants and products.
What is chemistry in school?
She has taught science courses at the high school, college, and graduate levels. Chemistry is a physical science that studies matter, energy and how they interact. When studying these interactions, it's important to understand the law of conservation of mass.
Who discovered the law of conservation of mass?
History credits multiple scientists with discovering the law of conservation of mass. Russian scientist Mikhail Lomonosov noted it in his diary as a result of an experiment in 1756. In 1774, French chemist Antoine Lavoisier meticulously documented experiments that proved the law. The law of conservation of mass is known by some as Lavoisier's Law.
Is mass constant in an isolated system?
Therefore, the mass contained in that isolated system will remain constant, regardless of any transformations or chemical reactions that occur—while the result may be different than what you had in the beginning, there can't be any more or less mass than what you had prior to the transformation or reaction. The law of conservation of mass was ...
Who is given credit for discovering the law?
Credit for discovering the law may be given to either Mikhail Lomonosov or Antoine Lavoisier.
Law of Conservation of Mass
The law of conservation of mass states that during a chemical reaction in a completely closed system, mass will not be created or destroyed. Further, the law of conservation of mass states is mass is conserved from reactants to products, no matter the type of chemical reaction that occurs.
Law of Conservation of Mass Examples
There are several ways to consider the law of conservation of mass examples. In a simple combination reaction where two substances are chemically combined, we will see conservation as follows: 300g A + 100g B --> 400g AB.
Law of Conservation of Mass Equation
Chemical reactions can be described in two ways: word equations and chemical equations. In a word equation, the names of the reactants and products are used instead of the chemical formulas. A chemical equation uses chemical formulas to illustrate what is happening in a reaction.
What is the sandwich illustration?
The sandwich illustration and interactive activity are intended to give students a real-life example of balancing to which they can relate. The idea is for students to see that the number and types of ingredients do not change during the “reaction” of making sandwiches; they only rearrange. A common misconception is that atoms of one type of element change into a different element during a reaction rather than rearranging to form new or different molecules. It is common sense to students that cheese does not turn into bread, and therefore, the engage activity should lay the foundation for addressing this misconception. Students could be asked for other examples from everyday life in addition to the sandwich example in order to help them visualize this concept. Some ideas are making s'mores, building a bike, or making a car (using tires, a steering wheel, doors, and a frame).
Why do we have multiples of a molecule?
In the simulation you just used, you saw that having multiples of a molecule is the way to increase the number of atoms needed for the equation to be balanced.
How do you know how many atoms are in a chemical?
So, how do you know how many atoms are present in a chemical? The rule is to take the coefficient (the number in front of the chemical formula) multiplied by the subscript (the number to the lower right of the element symbols) for each atom in the formula. If there is no number, it represents a 1. For example:
How many atoms are in H2O?
H2O has 2 atoms of hydrogen and 1 atom of oxygen. CaCl2 has 1 atom of calcium and 2 atoms of chlorine. 3C6H12O6 has 18 atoms of carbon, 36 atoms of hydrogen, and 18 atoms of oxygen. (Remember to multiply the coefficient 3 by each of the subscripts to get the total per atom.)
When an equation is written so that each type of atom has the same number of atoms in the reactants?
When an equation is written so that each type of atom has the same number of atoms in the reactants and products, we say the equation is balanced. For example:
How many sulfur atoms are there before a reaction?
Explain 1: Counting It Out. As you just saw in the videos, the number of each type of atom is the same before and after a reaction. If there are 5 sulfur atoms before a reaction, there will also be 5 sulfur atoms after the reaction. Atoms can’t just disappear!
What should students learn about conservation of mass?
It is vital that students master the concepts of subscripts and coefficients when count ing atoms.