What is the Lewis structure for CH3-?
What is the structure of CH3? (methyl carbanion) has tetrahedral structure (sp3) and one of the hybrid orbitals contains the lone pair of electrons. The carbanion has 3 bonding pairs and one lone pair. Thus, VSEPR theory predicts a tetrahedral geometry and a trigonal planer structure.
What is the Lewis structure for ch3ccch3?
In the lewis structure of CH 3–, there are three single bonds around the carbon atom, with three hydrogen atoms attached to it, and on the carbon atom, there is one lone pair. Also, there is a negative (-1) charge on the carbon atom. Here’s how you can draw the CH 3– lewis structure step by step.
What is the Lewis structure of ch3ch2ch3?
The lewis electron structure for the nh 4+ ion is as follows: Ch3ch2ch3 is the formula for propane. Propylamine has been identified in rice, corn, and barley (1) and occurs naturally in various species of marine algae (2).
How to make Lewis dot structure?
Method 2 Method 2 of 3: Creating Lewis Structures for Larger Covalent Molecules Download Article
- Determine which atom is your central atom. This atom is usually least electronegative. ...
- Consider the valence electrons of the central atom. As a general (but not all-exclusive) rule, atoms like to be surrounded by 8 valence electrons (the octet rule).
- Write the symbol of your central atom. ...
- Show the electron geometry of the central atom. ...
What is the molecular geometry of CH3CN?
0:043:08CH3CN Molecular Geometry, Bond Angles, and Polarity - YouTubeYouTubeStart of suggested clipEnd of suggested clipHere so it's bonded to two things there are no lone pairs all the electrons are in chemical bonds soMoreHere so it's bonded to two things there are no lone pairs all the electrons are in chemical bonds so zero lone pairs for this carbon right here it's linear with a bond angle of 180.
What bonds does CH3CN have?
A CH3CN molecule has 7 covalent bonds, 2 of which are pi bonds. The C atom bonded to the 3 H atoms has a tetrahedral geometry. The C atom bonded to the N atom has a linear geometry.
How many bonds does CH3CN have?
Acetonitrile CH3−C≡N contains 5 σ and 2 π bonds. There are three C-H σ bonds, one C-C σ sigma bond and on C-N σ bond. There are two carbon nitrogen π bonds.
How do you make CH3CN?
Preparation Of Acetonitrile - C2H3N or CH3CN By manufacturing acrylonitrile, it is obtained as a byproduct. It can also be synthesized by hydrogenation of mixtures of carbon monoxide or dehydration of acetamide and ammonia.
Does CH3CN have hydrogen bonding?
Water is capable of hydrogen bonding while CH3CN C H 3 C N is not since it has no hydrogen atoms bonded to oxygen, fluorine, or nitrogen (the requirement for hydrogen bonding).
Is CH3CN ionic or covalent?
These measures show that the CuCN and CuNC bonding is dominantly ionic while the bonding for CH3CN and CH3NC is dominantly covalent.
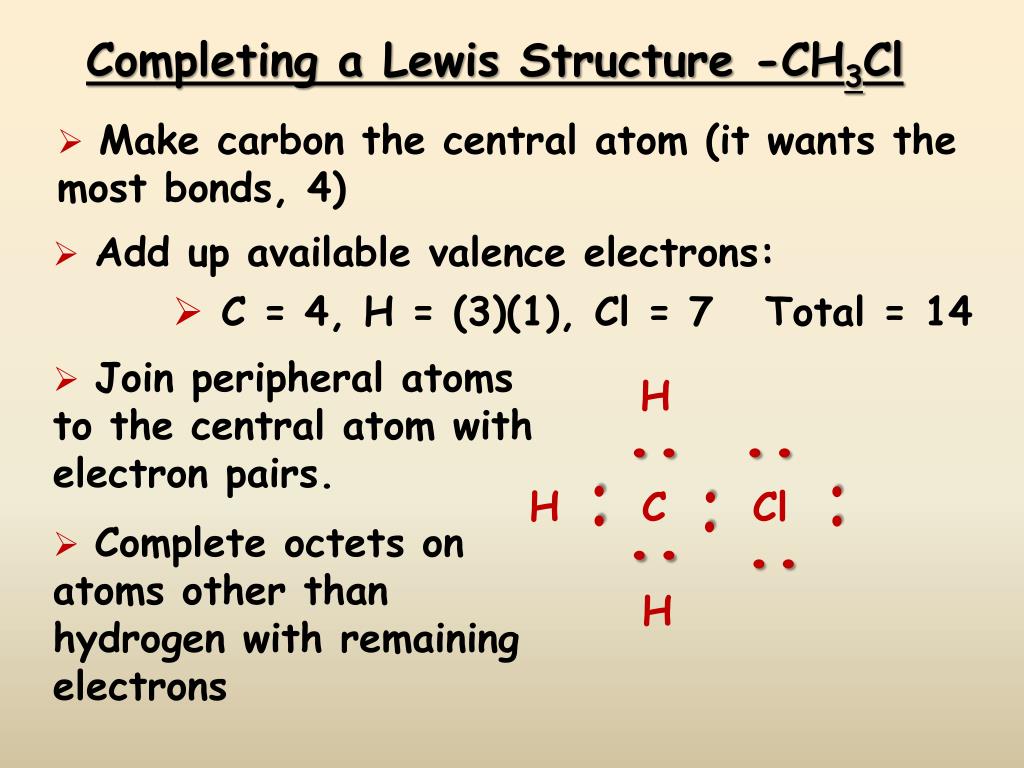
How do you draw Lewis structures?
How to Draw a Lewis StructureStep 1: Find the Total Number of Valence Electrons. ... Step 2: Find the Number of Electrons Needed to Make the Atoms "Happy" ... Step 4: Choose a Central Atom. ... Step 5: Draw a Skeletal Structure. ... Step 6: Place Electrons Around Outside Atoms. ... Step 7: Place Remaining Electrons Around the Central Atom.
How many atoms are bonded to C in CH3CN?
“Steric number is the addition of a total number of bonded atoms around a central atom and the lone pair present on it.” According to the CH3CN lewis structure, the left side carbon atom (C1) is bonded to three hydrogen atoms, one right side carbon(C2), and no lone pair present on it.
How many electrons does CH3CN have?
16 valence electronsFor CH3CN we have 4 valence electrons for the Carbon plus 1 for the Hydrogen (we have 3 Hydrogens) plus 4 for the other Carbon and then 5 for that Nitrogen, giving us a total of 16 valence electrons.
What is the hybridization of CH3CN?
Re: Hybridization of CH3CN The hybridization of the N is sp. This is because there are two region of electron density: the triple bond with the C and the lone pair.
What is the Lewis structure for c2h2?
0:012:24How to Draw the Lewis Dot Structure for C2H2: Acetylene (Ethyne)YouTubeStart of suggested clipEnd of suggested clipLet's draw the lewis structure for c2h2 this is acetylene. It's also called ethyne that's the iupac.MoreLet's draw the lewis structure for c2h2 this is acetylene. It's also called ethyne that's the iupac. Name to start out with lewis structures we count the total number of valence electrons.
Is CH3CN a dipole dipole?
CH3CN has dipole-dipole interaction forces but CH3CH2CH3 has no such intermolecular forces. Both CH3CN and CH3CH2CH3 are nonpolar.
Is CH3CN triple bond?
Classify the following: CH3CN. There is a triple bond between the C and N.
Is CH3CN a dipole dipole?
CH3CN has dipole-dipole interaction forces but CH3CH2CH3 has no such intermolecular forces. Both CH3CN and CH3CH2CH3 are nonpolar.
Is CH3CN polar?
The acetonitrile molecule is polar owing to the difference in the electronegativity of carbon and nitrogen atom due to which a slight negative charge develops on nitrogen while a slight positive charge on the carbon atom. What is this? The dipole moment of CH3CN is 3.5 Debye.
What is the type of hybridization in CH3CN?
In CH3CN, first carbon is sp3 hybridised (4 σ bonds). second carbon is sp hybridised (2 π bonds and 1 σ bond).
How many bonded pairs are there in CH3CN Lewis?
There is one lone pair on the nitrogen atom and a total of 7 bonded pairs present in the CH3CN lewis structure.
How many regions of electron density are there in CH3CN Lewis?
bonded atoms and lone pairs electrons. As we see in CH3CN lewis’s structure, the left side carbon has 4 regions of electron density and the right side carbon is 2 regions of electron density.
What is the molecular geometry of CH3CN?
The molecular geometry of CH3CN is either linear or tetrahedral depending on which central atom you have been considering as there are two carbon central atoms ( C1 and C2) present, therefore, their molecular geometry will be dependent on the region of the electron density.
Why is CH3CN polar?
Why CH3CN is polar? The nitrile functional group (C≡N) is polarized, because of the greater difference of electronegativity between carbon (2.55) and nitrogen (3.04), and the fact that a triple bond binds these 2 atoms. The consequence of this is the quite strong dipole moment (almost more than 3.5 Debye) in the CH3CN molecule.
What is the N of a carbon?
N denotes the lone pair on the central atom, according to the CH3CN lewis structure, No lone pair is present on the left side carbon. Hence, N = 0
How many hydrogens are in CH3?
Therefore, put the two carbon atoms adjacent to each other in a central position, and spread the three hydrogens around one carbon atom ( CH3 ), and set the nitrogen atom adjacent to other carbon ( C≡N ).
Which atom is the outer atom of CH3CN?
The outer atom in the CH3CN molecule is hydrogen and nitrogen, since, hydrogen atom only needs two electrons for completing the octet and it has already two electrons because of the single bond attached to it.
