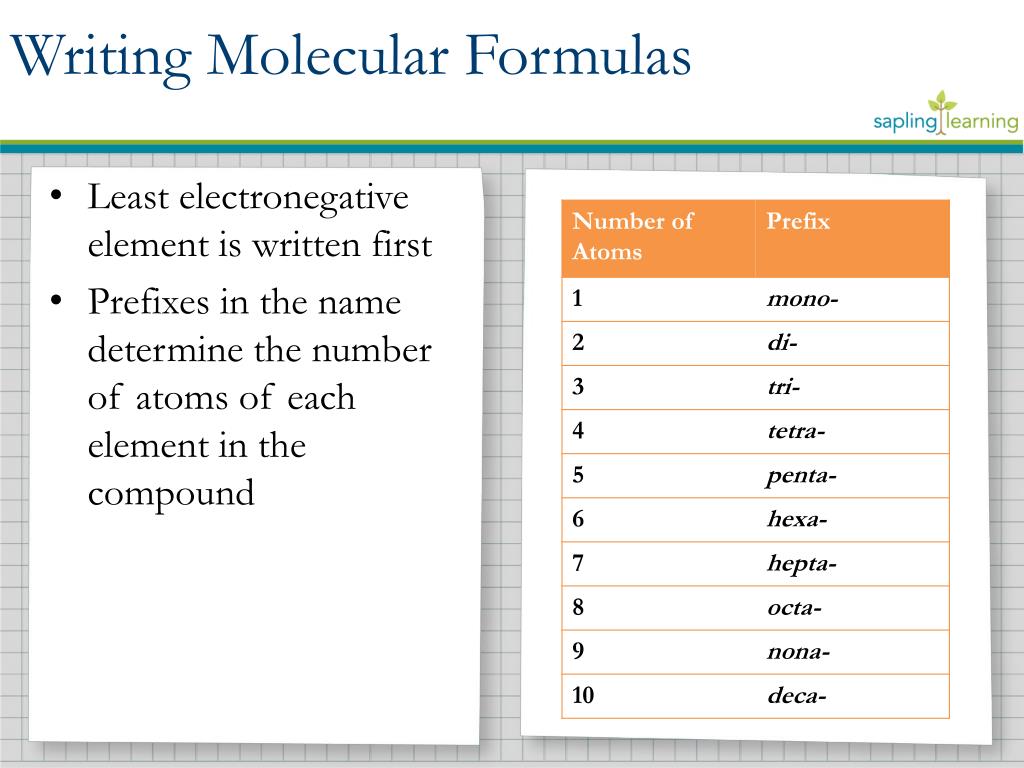
What is the smallest whole number ratio of components in a compound?
The smallest whole number ratio of components in a compound is found in empirical formulations. What shows a whole number ratio of the elements in a compound? formula based on actual data Empirical formulas are the lowest whole-number ratios of the components of a compound. Is the smallest possible ratio of the atoms in a compound?
What are ionic compounds composed of?
Although they are composed of ions, ionic compounds are electrically _____ neutral The lowest whole-number ratio of ions in an ionic compound is called a... formula unit
Which formula gives the smallest whole number ratio?
The empirical or simplest formula gives the smallest whole number ratio of elements in a compound, while the molecular formula gives the actual whole number ratio of elements. What is a smallest whole-number ratio? Why the empirical formula….
How do you find the ratio of elements in a compound?
Use each element’s molar mass to convert the grams of each element to moles. In order to find a whole-number ratio, divide the moles of each element by whichever of the moles from step 2 is the smallest. Which is the lowest ratio of elements in a compound the empirical or molecular formula?
What is the oxidation number of fluorine?
Can you name both ionic and covalent compounds?
About this website

What indicates the lowest whole number ratio of ions in an ionic compound?
formula unitA formula unit indicates the lowest whole number ratio of ions in an ionic compound. Because the positively and negatively charged ions in an ionic compound are arranged in a crystal lattice, there is no discrete particle comparable to a molecule.
What is the ratio of ions in an ionic compound?
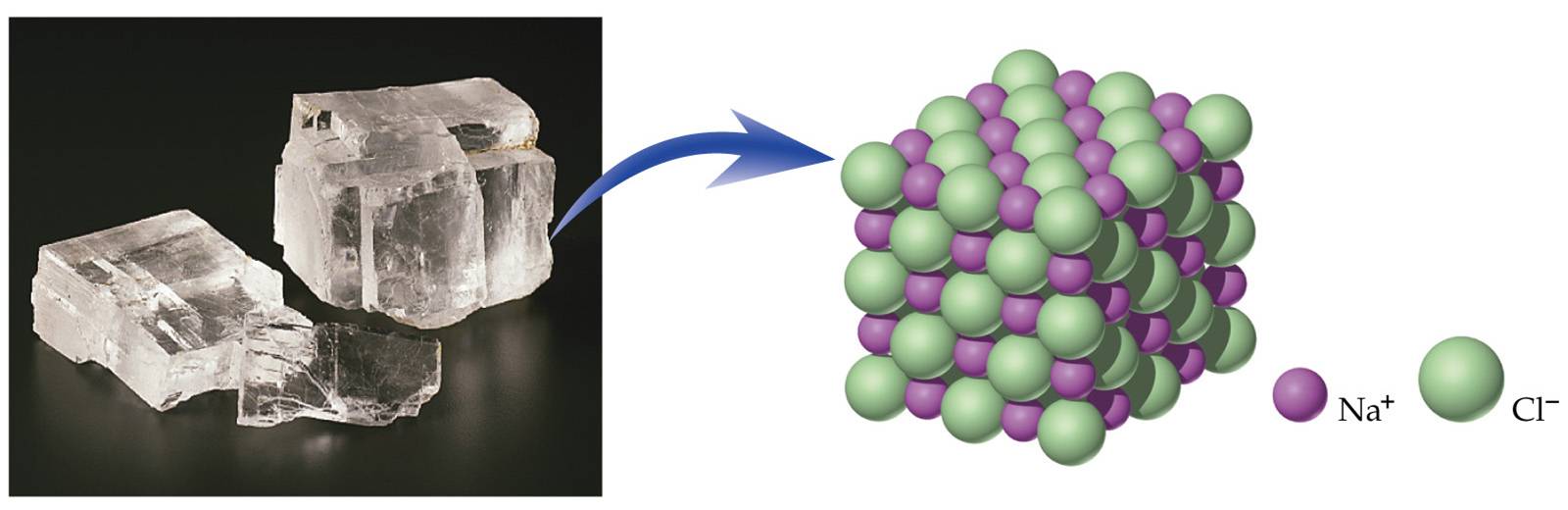
In the case of sodium chloride, the ratio of sodium ions to chloride ions, expressed in lowest whole numbers, is 1:1, so we use NaCl (one Na symbol and one Cl symbol) to represent the compound....Looking Closer: Blood and Seawater.IonPercent in SeawaterPercent in BloodHPO 4 2−, H 2PO 4 −—0.017 more rows•Jun 5, 2019
Which is the lowest ionic compound?
AgCl.The least ionic compound is .Therefore, is the least ionic compound among the given ionic compounds.
How do you find the ratio of ions?
The ion ratio is calculated as an intensity (or peak area) ratio of a less intense ion to that of a more intense ion. The reference ion ratio value is calculated as an average of ion ratios of calibration solutions.
How do you find the ratio of a compound?
Divide all elements by the smallest number of moles. This gives the ratio of atoms for each element in the compound.
Which of the following has the lowest ionic character?
Answer. Explanation: Agi has lowest ionic character.
Which bond has the least ionic character?
More is the difference in electronegativity between two elements , more is the ionic character in that bond or less is the covalent character. According to that reason, Br-Cl forms a bond with the least ionic character.
Which of the following is least ionic chloride?
Beryllium is extremely small in size and according to Fajan's rules, small highly charged ions tend to form covalent compounds. Hence, Be will form the least ionic chloride among all alkaline earth metals.
What is the ratio of ions in sodium oxide?
Sodium oxide molecules are made up of two sodium cations and one oxygen anion. Thus, Na2O molecules feature two sodium-oxygen ionic bonds.
What is the ratio of NaCl?
1:1 ratioSodium chloride , commonly known as salt (although sea salt also contains other chemical salts), is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions.
What are 4 properties of ionic compounds?
PROPERTIES OF IONIC COMPOUNDS They are usually crystalline solids. They have high melting points and high boiling points. They are usually soluble in water but insoluble in organic solvents. They conduct electricity when dissolved in water or when melted.
What is radius ratio rule in chemistry?
We define radius ratio as the ratio of radius of the cation to the radius of the anion. This ratio is very useful for determining the coordination number and the types of voids present in a given crystal.
What is the oxidation number of fluorine?
Be able to assign oxidation numbers to elements in a compound, as well as in polyatomic ions. Example: UF₆. - fluorine always has an oxidation number of -1. - multiply - 1 by 6. - the sum must equal zero so the total number of positive oxidation numbers is +6.
Can you name both ionic and covalent compounds?
Be able to use the Stock system to name both ionic AND covalent compounds (remember ionic compounds that contain transition metals will ALWAYS use the Stock system for naming no matter what).
What is the oxidation number of fluorine?
Be able to assign oxidation numbers to elements in a compound, as well as in polyatomic ions. Example: UF₆. - fluorine always has an oxidation number of -1. - multiply - 1 by 6. - the sum must equal zero so the total number of positive oxidation numbers is +6.
Can you name both ionic and covalent compounds?
Be able to use the Stock system to name both ionic AND covalent compounds (remember ionic compounds that contain transition metals will ALWAYS use the Stock system for naming no matter what).