
What two factors affect lattice energy?
Feb 03, 2020 · The lattice energy of CsCl is 633 kJ/mol. Click to see full answer. Regarding this, what is the lattice energy of LiCl? Lattice energy depends on the magnitudes of the charges of the ions and on the distance between them. Comparing LiCl, NaCl and KCl, we see that the lattice energies are: 860, 788, 699 kJ/mol, respectively.
How to solve lattice energy problems?
The lattice energy of CsCl is 633 kJ/mol. For CsCl the Madelung constant, M, is 1.763, and the Born exponent, n, is 10.7. The ionic radius of Cl– is known to be 1.81. How do you calculate lattice energy using Coulomb’s law?
Which one has the greater lattice energy?
The magnitude of the lattice energy for CsCl is a measure of the stability of cubic close-packed structures. It can be calculated by dividing its total kinetic energy by 3*N^2, where N is the number of atoms in an individual cell. The equation to calculate it is as follows: Magnitude or Lattice Energy=\frac{3N^{2}}{\sqrt3}
How can you predict lattice energy?
Nov 25, 2012 · What is CsCl? CsCl is the chemical formula of caesium chloride. What is the lattice energy for NaCl? The lattice energy of sodium chloride is 789 kJ/mol. What is …
How do you find the lattice energy of CsCl?
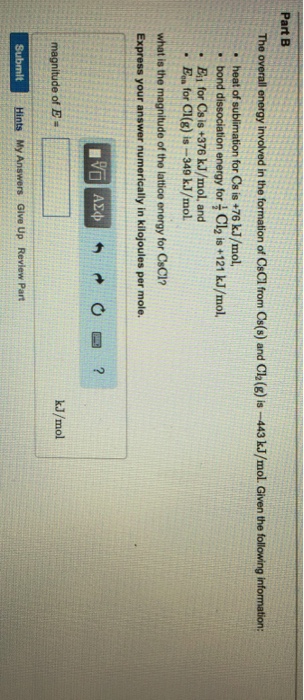
Estimate the lattice energy using a Born-Haber cycle.Cs(s) + ½ Cl2(g) → CsCl(s) ΔHf° = –433 kJ/mole.Cs(s) → Cs(g) S = 79 kJ/mole.Cs(g) → Cs+(g) + e– IE = 375 kJ/mole.½ Cl2(g) → Cl(g) ½BDE = ½×242 = 121 kJ/mole.Cl(g) + e– → Cl–(g) EA = 349 kJ/mole.Cs+(g) + Cl–(g) → CsCl(s) ElatMar 28, 2019
How do you find the magnitude of lattice energy?
0:5413:29Lattice Energy of Ionic Compounds, Basic Introduction, Charge vs Ionic ...YouTubeStart of suggested clipEnd of suggested clipNow lattice energy depends on the charge q1 q2 thus the charges of the ions. And. It's alsoMoreNow lattice energy depends on the charge q1 q2 thus the charges of the ions. And. It's also dependent on the distance between the ions. So as you increase the charge of the ion.
Does CsCl have high lattice energy?
CsCl has more ionic bonds than NaCl. The lattice energy is directly proportional to the ion charge and inversely proportional to the radius of the atom. The radius of Cs+ is now much greater than Na+ between NaCl and CsCl, even though CsCl is more ionic, but there is still more radius and thus less lattice energy.
What is the lattice energy of cesium?
The Born–Haber CycleSubstanceΔHsub (kJ/mol)Cs76.5Be324.0Mg147.1Ca177.87 more rows•Feb 28, 2021
How is lattice energy calculation Born Haber cycle?
The net enthalpy of formation and the first four of the five energies can be determined experimentally, but the lattice energy cannot be measured directly. Instead, the lattice energy is calculated by subtracting the other four energies in the Born–Haber cycle from the net enthalpy of formation.
Which compound has the largest magnitude of lattice energy?
Smaller the size of ions, larger the magnitude of charges, more the lattice energy. thus, AlN has the largest lattice energy.
What is the lattice energy of calcium fluoride?
The value of lattice energy of MgF2, CaF2 and ZrO2 molecules are, -2913 Kj/mole , -2609 Kj/mole and- 8714.5 kJ/ mole respectively. In case of molecule, the extent of charge on both 'Mg' and' Ca' metal is, +2 and the extent of charge on 'F atom is, -1 . That is, both cations and anions contain same extent of charge.
Why is CsCl lattice more stable than NaCl?
CsCl is more stable than NaCl. This is because higher the coordination number, greater are the forces of attraction between the cations and anions in the close-packed arrangement. As CsCl has co-ordination number of 8:8 while NaCl has a coordination number of 6 :6 , therefore, CsCl is more stable.Mar 12, 2022
Why is CsCl ionic?
0:041:14Is CsCl (Cesium chloride) Ionic or Covalent/Molecular? - YouTubeYouTubeStart of suggested clipEnd of suggested clipWe have a metal and a non-metal that's considered ionic so we're expecting this to be an ionicMoreWe have a metal and a non-metal that's considered ionic so we're expecting this to be an ionic compound let's look at the difference in electronegativity between the cesium.
What is the lattice energy of MgCl2?
The CRC Handbook of Chemistry and Physics lists the MgCl2 lattice energy as 2540 kJ mol−1 .
What is the lattice energy of RbCl?
Calculate the Lattice Energy (Enthalpy of formation) for Rubidium Chloride, given: ΔHof[Rb(g)]=80.9kJ/mol. ΔHof[RbCl(s)]=−435.4kJ/mol.Sep 3, 2021
Physics
what forms of energy are involved in the following situations? (choices: kinetic energy, potential energy - gravitational potential energy, elastic potential energy) a. a bicycle coasting along a level road b. heating water c.
ap chem
calculate the lattice enthalpy of potassium fluoride from the following data: enthalpy of formation of K (g): +89 kJ · mol−1 first ionization energy of K (g): +418 kJ · mol−1 enthalpy of formation of F (g): +79 kJ · mol−1
science
An antacid tablet added to a glass of water fizzes and bubbles as it mixes. Which of the indicators of a chemical change is represented in this scenario? formation of a gas change in color change in energy formation of a
Science
Hydrogen gas (H2) and chlorine gas (CL2) react to form hydrocloric acid (HCL). What is the correct balanced chemical equation for this reaction? A-2H2+2CL2->HCL B-H2+CL2->2HCL C-2H2+CL2->2HCL D-H2+CL2->HCL I think C Pls Help me
chemistry
Write the half reaction and balanced equation for: Cl2 (g) + H2 (g) -> HCl (aq) so I got: 2e- + Cl2 (g) -> 2Cl- H2 (g) -> 2H+ + 2e- so Hydrogen is oxidized and Chlorine is reduced so for cell notation I put:
Chemistry
For the reaction shown, compute the theoretical yield of product (in grams) for each of the following initial amounts of reactants. 2Al (s) +3 Cl2 (g) ---> 2 AlCl3 (s) A. 2.5gAl , 2.5g Cl2 B. 7.7 g Al , 25.2 g Cl2 C. 0.240 g Al ,