
Difference Between SN1 and SN2:
| SN1 | SN2 |
| SN1 is a unimolecular reaction | SN2 is a bimolecular reaction |
| It follows a 1st order kinetic mechanism ... | It follows the 2nd order Kinetic mechani ... |
| SN1 involves two steps | SN2 is a single-step process |
| In SN1, the rate of reaction depends on ... | The rate of reaction depends on the conc ... |
What are the factors that affect SN1 and SN2 reaction?
Factors affecting SN1 and SN2 reactions. Nature of substrate; The nucleophilicity of the reagents; Solvent polarity ; The alkyl halide and leaving group structures must be taken into account when calculating the unimolecular transition state of SN1. The formation of stable carbocations by alkyl halides with SN1 is easier than for alkyl halides ...
What are the mechanisms evolved on SN1 reaction?
SN1 Reaction Mechanism can Include the Following: Formation of Carbocation, Nucleophilic Attack, Deprotonation of Nucleophile. Learn about SN1 Reaction Mechanism
How do SN1 reactions differ from SN2 reactions?
The key difference between SN1 and SN2 reactions is that SN1 reactions have several steps whereas SN2 reactions have only one step. What are SN1 Reactions? In SN1 reactions, 1 indicates that the rate determining step is unimolecular. Thus, the reaction has a first-order dependence on electrophile and zero-order dependence on nucleophile.
Why is a weak nucleophile used in a SN1 mechanism?
Why is a weak nucleophile used in an SN1 mechanism? Weak nucleophile is used in SN1 because it contains excess of solvent (polar protic) which is used in ionisation (due to dipole-dipole interaction, leaving group easily goes out) and thereby forms carbocation.
What is the stereochemistry of SN1?
What is SN in chemistry?
What is the name of the reaction in which a nucleophile attacks a halogen atom?
Which atom is attacked by the nucleophile?
Which is more stable, a tertiary or secondary carbocation?

What are SN1 and SN2 reactions mechanism?
In SN1, the rate of reaction depends on the concentration of the substrate. The rate of reaction depends on the concentration of both the substrate and the nucleophile. In SN1 as the leaving group leaves, the substrate forms a carbocation intermediate. In SN2 the reaction happens in a single transition state.
What is the mechanism of SN1 reaction?
SN1 reaction mechanism follows a step-by-step process wherein first, the carbocation is formed from the removal of the leaving group. Then the carbocation is attacked by the nucleophile. Finally, the deprotonation of the protonated nucleophile takes place to give the required product.
What is the mechanism of SN2 reaction?
The SN2 reaction mechanism involves the nucleophilic substitution reaction of the leaving group (which generally consists of halide groups or other electron-withdrawing groups) with a nucleophile in a given organic compound.
How do you know if a mechanism is SN1 or SN2?
0:387:42Choosing Between SN1 and SN2 Reactions (vid 1 of 2) By Leah4sciYouTubeStart of suggested clipEnd of suggested clipAnd finally for solvents if we have a polar protic solvent. We can easily dissolve any charges thatMoreAnd finally for solvents if we have a polar protic solvent. We can easily dissolve any charges that form and this tends to favor and so1 reaction.
Is SN2 first or second order?
The term SN2stands for Substitution reaction, Nucleophilic, 2nd order (also called bimolecular). According to the SN2 mechanism, there is a single transition state because bond-breaking and bond-making occur simultaneously.
What is SN1 mechanism explain with suitable example?
The order of reaction is one. The hydrolysis of tert-butyl bromide with aqueous NaOH solution is an example of SN1 reaction. The rate of the reaction depends on the concentration of tert butyl bromide but it is independent of the concentration of NaOH. Hence, the rate determining step only involves tert-butyl bromide.
What is the difference between SN1 and SN2?
SN1 and SN2 reactions are two nucleophile substitution reactions in which SN1 involves only one molecule whereas SN2 reaction involves two molecules.
Is SN1 first or second order?
The Rate Law Of The SN1 Reaction Is First-Order Overall.
Why is it called SN1?
The SN1 reaction is a substitution reaction in organic chemistry, the name of which refers to the Hughes-Ingold symbol of the mechanism. "SN" stands for "nucleophilic substitution", and the "1" says that the rate-determining step is unimolecular.
How do you draw SN1 mechanisms?
10:2216:4889: Drawing the SN1 mechanism - YouTubeYouTubeStart of suggested clipEnd of suggested clipThe alkyl halide make sure that it's capable of sn1. Look at the carbon that's holding the leavingMoreThe alkyl halide make sure that it's capable of sn1. Look at the carbon that's holding the leaving group it should have 1 or 0 hydrogen's. It has 0.
How do you know if a reaction is E1 or E2?
The most obvious way to distinguish E1 vs E2 is by looking at the number of steps in the mechanism. E1 takes place in two steps and has a carbocation intermediate; on the other hand, E2 takes place in one step and has no intermediate.
What does S N 1 mean?
Remember, S N 1 means the leaving group leaves and only after that the nucleophile attacks. That is the definition of the S N 1 mechanism, it is a unimolecular mechanism, i.e. only one of the reactants (in this case the substrate) participates in the rate determining step: What you need to remember, to understand the problem with this mechanism, ...
What is the mechanism of a strong nucleophile?
For secondary substrates, the mechanism is determined largely by the nucleophile and the principle here is that: a strong nucleophile does SN2, while a weak nucleophile does SN1. In simple words, a strong nucleophile means a reactive/aggressive/unstable nucleophile, so one that is has a large electron density ...
Which two nucleophiles are involved in substitution reactions?
The two main nucleophiles are the water and alcohols . In addition to the nucleophile, the solvent also plays a role in determining the major mechanism in nucleophilic substitution reactions. Here, you need to remember that polar aprotic solvents favor the SN2 mechanism, while polar protic solvents favor the SN1 mechanism.
Is a primary carbocation unstable?
What you need to remember, to understand the problem with this mechanism, is that, primary carbocations are very unstable and they are simply not formed under normal circumstances. So, this mechanism is impossible: The only way to achieve a substitution is to involve the nucleophile and substrate at the same time in the rate determining step: ...
SN1 Reaction - definition
In the SN1 reaction, a planar carbenium ion is formed first, which then reacts further with the nucleophile. Since the nucleophile is free to attack from either side, this reaction is associated with racemization.
Reactivity towards SN1 and SN2 - example
The SN 2 tends to proceed with strong nucleophiles; by this, generally means negatively charged nucleophiles such as CH 3 O (-), CN (-), RS (-), N_3 (-), HO (-), and others. The SN 1 tends to proceed with weak nucleophiles generally neutral compounds such as solvents like CH 3 OH,H 2 O,CH 3 CH 2 OH, and so on.
What is Sn1 reaction?
Ans. Sn1 is a unimolecular substitution reaction. It involves the separation of negatively charged functional groups or atoms first. This results in the formation of a carbocation. The anion or another negatively charged functional group or atoms then gets attached to the carbocation.
What is the difference between Sn1 and Sn2?
A nucleophilic substitution reaction is a reaction that involves the replacement of one functional group or atom with another negatively charged functional group or atom. Sn1 is a unimolecular reaction while Sn2 is a bimolecular reaction. Sn1 involves two steps. Sn2 involves one step.
How many steps does Sn1 have?
Sn1 involves two steps. Sn2 involves one step. In Sn1, there is a stage where carbocation forms. The anion or the negatively charged atoms or compounds then gets attracted to the carbocation. In Sn2, there is only a transition stage and no formation of intermediates.
What is a nucleophilic substitution reaction?
Nucleophilic Substitution Reaction. Any substitution reaction that involves replacing of an atom or a functional group by a negatively charged ion or by an atom or functional group that has a lone pair of electrons.
How many molecules are involved in SN1 and SN2?
SN1 involves one molecule while Sn2 involves two molecules. To understand SN1 and SN2, it is imperative to know what a nucleophilic substitution reaction is. Only after one gets to understand all the terminologies pertaining to the nucleophilic substitution reaction, understanding the difference between Sn1 and Sn2 becomes easier.
Why is substitution called nucleophilic?
It is called nucleophilic because of the involvement of negatively charged atoms or molecules. 2.
What is the slow step in Sn1?
This is known as the slow step or the rate-limiting step. As the Br ion gets separated, the CN negative ion around gets attracted to CH3 and it attacks CH3 to form CH3CN. Furthermore, the first step is considered the main step in Sn1. Since the first step involves only one kind of molecule, it is a unimolecular reaction.
Which solvent is more likely to follow the S N 1 pathway?
The more highly substituted is the incipient carbenium ion, the more probable that the reaction will follow an S N 1 pathway.
Which group stabilizes an incipient negative charge?
Very good leaving groups, such as triflate, tosylate and mesylate, stabilize an incipient negative charge. The delocalization of this charge is reflected in the fact that these ions are not considered to be nucleophilic.
What is nucleophilic substitution?
Nucleophilic substitution is the reaction of an electron pair donor (the nucleophile, Nu) with an electron pair acceptor (the electrophile). An sp 3 -hybridized electrophile must have a leaving group (X) in order for the reaction to take place.
What is the role of polar protic solvents in reducing reactivity?
An additional factor that plays a role is the character of the solvent. Increasing stabilization of the nucleophile by the solvent results in decreasing reactivity. Thus, polar protic solvents will stabilize the chloride and bromide ions through the formation of hydrogen bonds to these smaller anions.
What is the mechanism of SN1?
Mechanism: SN1 Reactions: SN1 reactions have several steps; it starts with the removal of the leaving group, resulting a carbocation and then the attack by the nucleophile. SN2 Reactions: SN2 reactions are single step reactions where both nucleophile and substrate are involved in the rate determining step. Therefore, the concentration of the ...
What is the difference between SN1 and SN2?
The key difference between SN1 and SN2 reactions is that SN1 reactions have several steps whereas SN2 reactions have only one step.
What solvents are SN1 and SN2?
They can also act as the nucleophiles for the reaction. SN2 Reactions: SN2 reactions proceed well in polar aprotic solvents such as acetone, DMSO, and acetonitrile.
What does SN1 mean?
The two symbols SN1 and SN2 refer to two reaction mechanisms. The symbol SN stands for “nucleophilic substitution”. Even though both SN1 and SN2 are in the same category, they have many differences including the reaction mechanism, nucleophiles and solvents participated in the reaction, and the factors affecting the rate determining step. ...
How is carbocation formed in SN1?
SN1 reactions have three steps. Formation of the carbocation by removing the leaving group. The reaction between the carbocation and the nucleophile (Nucleophilic attack).
Which is the most stable barrier in SN1?
The rate of the reaction is proportional to the stability of the carbocation. Therefore, the formation of the carbocation is the greatest barrier in SN1 reactions. The stability of the carbocation increases with the number of substituents and the resonance. Tertiary carbocations are the most stable and primary carbocations are the least stable ...
What is the stereochemistry of SN1?
Stereochemistry of SN1 reaction: In SN 1 reaction, carbocations are formed as the intermediate which are trigonal and planar. Carbocation has a flat structure so that nucleophile can attack it from either side (i.e. front or back) resulting in the formation of two products, one with retention of configuration and other with inversion ...
What is SN in chemistry?
Nucleophilic substitution (SN) reaction. Nucleophiles are electron rich atoms or group of atoms which attack on electron deficient centre during chemical reaction. Nucleophiles are the negatively charged species or neutral species having electron rich centre. Any substitution reaction that involves replacing of an atom or a functional group by ...
What is the name of the reaction in which a nucleophile attacks a halogen atom?
During nucleophilic substitution reaction in haloalkanes (alkyl halides) , the nucleophile attacks the haloalkane and replaces the halogen atom.
Which atom is attacked by the nucleophile?
It is assumed that the nucleophile attacks the carbon atom attached to the halogen atom from the side opposite to the halogen (i.e. backside attack). As a result a transition state (activated complex) is formed in which carbon atom is partially bonded to both nucleophile and leaving group (halogen atom).
Which is more stable, a tertiary or secondary carbocation?
A tertiary carbocation is more stable than a secondary carbocation which is more stable than a primary carbocation. Greater the stability of the carbocation, greater will be the ease of formation of carbocation, and hence faster will be the rate of the reaction.
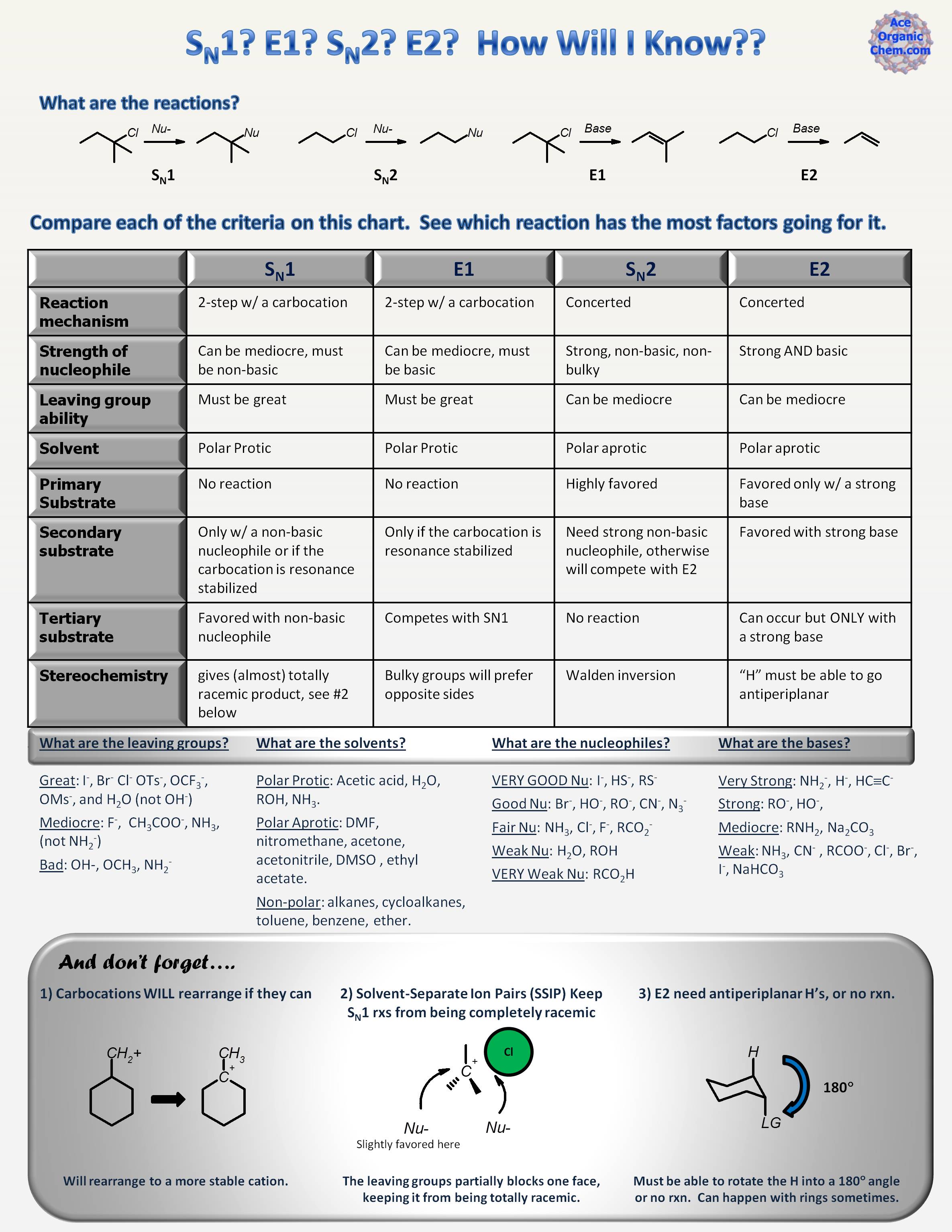
Is It An SN1 Or SN2 Mechanism?
Why Can’T Primary Do Sn1?
Why Can’T Tertiary Do SN2?
- Now let’s see why tertiary substrates can undergo SN1 reactions only. If you were to do an SN2 mechanism on a tertiary substrate you’d have to show the nucleophile attacking the substrate while the leaving group is still there (by definition of the SN2 mechanism). And again, let’s draw a hypothetical mechanism for this: The problem here is that the nucleophile does not have space t…
What About The Secondary Substrates?
- Now the secondary substrate – the troublemaker. For secondary substrates, the mechanism is determined largely by the nucleophile and the principle here is that: a strong nucleophile does SN2, while a weak nucleophile does SN1. In simple words, a strong nucleophile means a reactive/aggressive/unstable nucleophile, so one that is has a large electron density (lone pairs …
Determining SN1 Or SN2 Based on The Stereochemistry of The Product
- In some cases, you may be asked to determine the mechanism based on the structure, mainly the stereochemistry, of the product. Two important features you need to remember for this: 1. SN2 mechanism goes through inversion of configuration 2. SN1 mechanism goes through the racemization Here, we are talking about the absolute configuration of the elec...