
What are the main features of the modern periodic table?
Main features of the Modern Periodic Table . The modern periodic table contains 7 horizontal rows called periods and 18 vertical columns called groups. Apart from the seven rows, there are two additional rows placed separately at the bottom of the table.
How would you describe the modern periodic table?
Summary
- The periodic table is arranged in order of atomic number
- A period is a horizontal row of the periodic table.
- A group is a vertical row of the periodic table.
What two properties are the modern periodic table based on?
What two properties are the modern periodic table based on? The modern table is based on Mendeleev's table, except the modern table arranges the elements by increasing atomic number instead of atomic mass. Atomic number is the number of protons in an atom, and this number is unique for each element. Click to see full answer.
What were the drawbacks of modern periodic table?
The disadvantages of modern periodic table are. Position of hydrogen is not satisfactory, it can be placed either in group I or 17 group of the first period. It fails to accommodate lathanides and actinides in the main body of the periodic table. These are ….
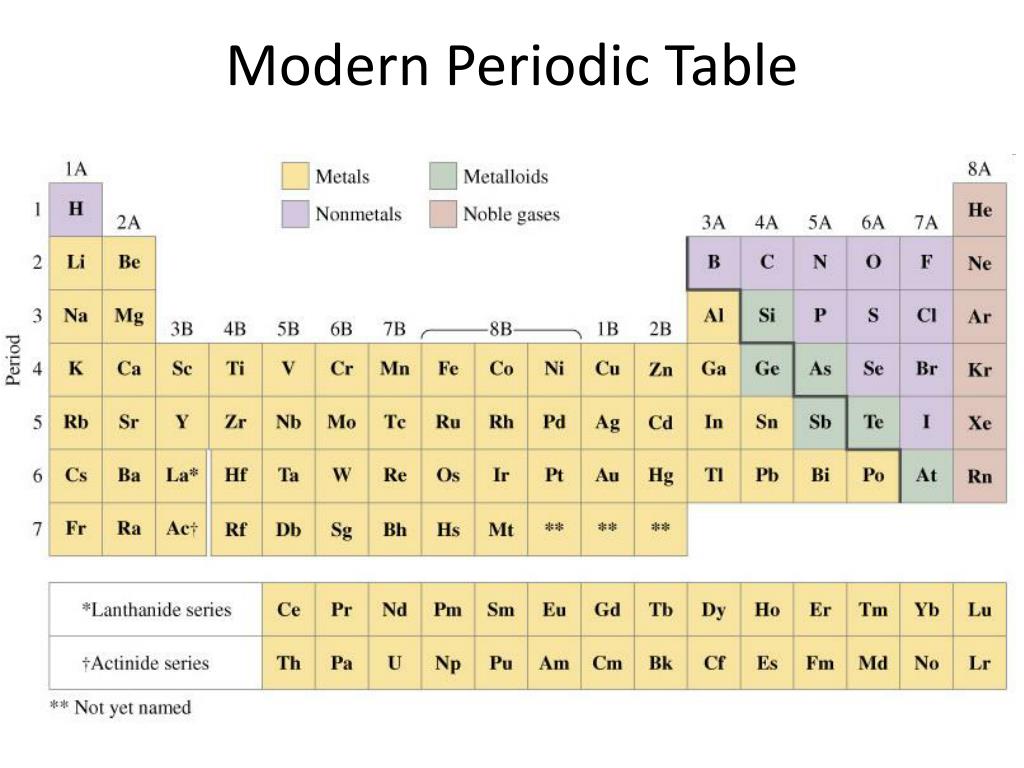
What is the modern periodic table called Class 10?
Thus, in the modern periodic table, atomic number forms the basis of the classification of elements. The modern table is called 'long form' of the periodic table.
Why is it called periodic table?
Why is the periodic table called the periodic table? It is called the periodic table because of the way the elements are arranged. You'll notice they're in rows and columns. The horizontal rows (which go from left to right) are called 'periods' and the vertical columns (going from up to down) are called 'groups'.
Why is modern periodic table is known as Long Form?
The Modern Periodic Table is called as "long form" of the periodic table,since in comparison to Mendeleev's Periodic table,it is longer and have the capacity to not only accommodate those elements which have been discovered but also which are yet to be discovered.
Are there in modern periodic table?
The modern periodic table is the present form of the periodic table in which the arrangement of elements is in the increasing order of their atomic numbers. There are 18 vertical columns or groups and 7 horizontal rows or periods in the modern periodic table.
Who Made modern periodic table?
Dmitri MendeleevAlbert GhiorsoPeriodic table/Inventors
Who gave the modern periodic law?
Who gave the modern periodic law? In 1869, Dmitri Mendeleev and Lothar Meyer established the periodic law independently.
What is the difference between periodic table and modern periodic table?
Is modern periodic table different from general periodic table?...Mendeleev's periodic tableModern periodic table1. Mendeleev's periodic table is based on atomic mass.1. The modern periodic table is based on the atomic number.4 more rows
Which is the longest modern periodic table?
The sixth periodThe sixth period is called the longest period because it has maximum 32 elements.
What is modern periodic table used for?
Scientists use the periodic table to quickly refer to information about an element, like atomic mass and chemical symbol. The periodic table's arrangement also allows scientists to discern trends in element properties, including electronegativity, ionization energy, and atomic radius.
What group is the modern periodic table in?
The elements of groups 1, 2, 13, 14, 15, 16, and 17 are known as the main group elements or normal elements. The elements of groups 3, 4, 5, 6, 7, 8, 9, 11 and 12 are known as the transition elements. Group 18 is called noble gases or inert gases. Their outermost shell is completely filled.
What is difference between Mendeleev and modern periodic table?
Mendeleev's Periodic Law states that the physical and chemical properties of elements are periodic functions of their atomic weights. On the other hand, the Modern periodic Law states that the physical and chemical properties of elements are periodic functions of their atomic numbers.
What is the modern atomic?
Atoms are no longer indivisible and consist of electrons, protons, neutrons and even more sub-particles. Atoms of the same element may differ from one another called isotopes. Atoms of different elements may be similar called isobars.
Who gave the periodic table its name?
Mendeleev discovered the periodic table (or Periodic System, as he called it) while attempting to organise the elements in February of 1869.
Who is periodic table named after?
Dmitri MendeleevHowever, it is distinguished in being named aftet the scientist most frequently associated with the periodic table. Dmitri Mendeleev is credited with coming up with the first iteration of the modern periodic table back in 1869.
What does a the periodic table stand for?
periodic table, in full periodic table of the elements, in chemistry, the organized array of all the chemical elements in order of increasing atomic number—i.e., the total number of protons in the atomic nucleus.
Which elements belong to group 15 of the modern periodic table?
In the modern periodic table of elements, the following fall under group 15: Nitrogen (N) Phosphorus (P) Arsenic (As) Antimony (Sb) Bismuth (Bi) Th...
What trends in electronegativity can be seen in the modern periodic table of elements?
In the modern periodic table, the electronegativity of elements increases across a period (row) and decreases down a group (column). Therefore, the...
How are the elements classified into different blocks in the modern periodic table?
The modern periodic table of elements can be broken down into 4 blocks – the s-block, the p-block, the d-block, and the f-block. This classificatio...
What are the f-block elements?
The f-block elements are the elements of the modern periodic table whose valence electrons lie in f-orbitals. These elements can be broadly classif...
What are the trends in the atomic/ionic radii of elements in the modern periodic table?
The atomic/ionic radii of elements increase while traversing down a group in the modern periodic table due to the addition of new electron shells....
What is the periodic table?
The periodic table is a tabular array of the chemical elements organized by atomic number, from the element with the lowest atomic number, hydrogen...
What do periodic table groups have in common?
The groups of the periodic table are displayed as vertical columns numbered from 1 to 18. The elements in a group have very similar chemical proper...
Where does the periodic table come from?
The arrangement of the elements in the periodic table comes from the electronic configuration of the elements. Because of the Pauli exclusion princ...
Why does the periodic table split?
The periodic table has two rows at the bottom that are usually split out from the main body of the table. These rows contain elements in the lantha...
Why do atomic radii decrease?
The atomic radii of the elements generally decrease across periods due to the increase in electronegativity and the increase in the effective nuclear charge acting on the outermost shells.
What is the f block?
The f-block elements are the elements of the modern periodic table whose valence electrons lie in f-orbitals. These elements can be broadly classified into two categories.
What are the f block elements?
The f-block elements are the elements of the modern periodic table whose valence electrons lie in f-orbitals. These elements can be broadly classified into two categories. The lanthanides – elements whose valence electrons lie in the 4f orbital. The actinides – elements whose valence electrons lie in the 5f orbital.
What are the periods of the periodic table?
When we talk about the periods of a modern periodic table, one should keep in mind that the number of shells present in an atom determines its period number. The elements of period one will have only one shell, elements of period two will have two shells and so on. The first period of the modern periodic table is the shortest period as it contains only two elements. The period number two and three consists of eight elements each and is known as short groups. Period four and five have eighteen elements and are known as the long group. In the modern periodic table, group number 3 of period six contains the lanthanide series which are the rare earth elements. We have radioactive elements (actinides) present in group 3 of period seven.
Why was the modern periodic table rejected?
This method was rejected as it could not explain the position of certain elements, rare earth metals, and isotopes. A scientist named Henry Moseley removed these defects and put forward the modern periodic table with the modern periodic law.
What is a tabular arrangement of elements in groups and periods which highlights the regular trends in properties of elements?
A tabular arrangement of elements in groups and periods which highlights the regular trends in properties of elements is defined as the periodic table.
How many elements are in a vertical column?
Earlier scientists assumed that the properties of elements are periodic functions of their atomic masses. On the basis of this assumption, Mendeleev placed 63 elements in a vertical column called groups and in horizontal rows called periods.
What are the rows of lanthanoid and actinoid?
These rows contain elements in the lanthanoid and actinoid series, usually from 57 to 71 ( lanthanum to lutetium) and 89 to 103 ( actinium to lawrencium ), respectively. There is no scientific reason for this. It is merely done to make the table more compact.
What is the atomic number of an element?
The atomic number of an element is the number of protons in the nucleus of an atom of that element . Hydrogen has 1 proton, and oganesson has ...
What is the periodic table?
periodic table, in full periodic table of the elements, in chemistry, the organized array of all the chemical elements in order of increasing atomic number —i.e., the total number of protons in the atomic nucleus. When the chemical elements are thus arranged, there is a recurring pattern called the “periodic law” in their properties, ...
What elements are triads?
Döbereiner in 1817 showed that the combining weight, meaning atomic weight, of strontium lies midway between those of calcium and barium, and some years later he showed that other such “ triads ” exist (chlorine, bromine, and iodine [halogens] and lithium, sodium, and potassium [alkali metals]). J.-B.-A. Dumas, L. Gmelin, E. Lenssen, Max von Pettenkofer, and J.P. Cooke expanded Döbereiner’s suggestions between 1827 and 1858 by showing that similar relationships extended further than the triads of elements, fluorine being added to the halogens and magnesium to the alkaline-earth metals, while oxygen, sulfur, selenium, and tellurium were classed as one family and nitrogen, phosphorus, arsenic, antimony, and bismuth as another family of elements.
Why do the elements in the periodic table have different orbits?
The arrangement of the elements in the periodic table comes from the electronic configuration of the elements. Because of the Pauli exclusion principle, no more than two electrons can fill the same orbital. The first row of the periodic table consists of just two elements, hydrogen and helium. As atoms have more electrons, they have more orbits available to fill, and thus the rows contain more elements farther down in the table.
How many protons does hydrogen have?
The atomic number of an element is the number of protons in the nucleus of an atom of that element. Hydrogen has 1 proton, and oganesson has 118.
What are the elements that are related to the first seven?
Newlands proposed classifying the elements in the order of increasing atomic weights, the elements being assigned ordinal numbers from unity upward and divided into seven groups having properties closely related to the first seven of the elements then known: hydrogen, lithium, beryllium, boron, carbon, nitrogen, and oxygen . This relationship was termed the law of octaves, by analogy with the seven intervals of the musical scale.
What is the Moseley periodic law?
Moseley's Periodic Law. Henry Moseley showed that the chemical and physical properties of elements is determined by the atomic number and not by the atomic mass. He restated the periodic law as-. 'Physical and chemical properties of an element are a periodic function of its atomic number.'. The Moseley's periodic law is also known as ...
What is the name of the column in which the elements are arranged?
It was only in 1869 that Mendeleev published his paper in the Journal of Russian Chemical Society where he classified the elements based on their atomic masses and arranged them into horizontal rows called periods and vertical columns called groups . The periodic law states that the properties of elements are a periodic function of their relative atomic masses. Mendeleev was successful in arranging all 63 elements that were known in his time into a tabular form which contained eight columns and seven rows. It also contained some gaps which were later filled after the discovery of new elements.
What did Johann Döbereiner do?
He grouped the elements into gases, non-metals, metals and earthly elements. After several decades, in 1829 Johann Döbereiner made an attempt to group elements. Based on the similarity in chemical properties, he grouped elements into triads.
How many rows are there in the periodic table?
Following are some of the main features of modern periodic table. Elements are grouped in ascending order of their respective atomic number. There are seven horizontal rows called periods and eighteen vertical columns called groups.
Where are transition metals placed on the periodic table?
Some of the transition metals are placed separately in two rows at the bottom of the periodic table. These are known as Lanthanides and Actinides. Metalloids and non-metals: Metalloids generally appear in a diagonal line at the right side of the periodic table.
How do elements change properties in a period?
The elements in a period show a gradual change in properties on moving from left to right. Atomic size gradually decreases as we move from left to right.
Which group of elements is the left side of the periodic table?
Alkali and Alkaline Earth metals: The first two groups on the left side of the periodic table consists of highly reactive elements (except hydrogen). The first group elements contain one electron in the valence shell while the second group elements contain two electrons in their valence shell. Transition metals: These elements occupy the centre ...
What is Modern Periodic Law?
The modern periodic table is developed after the periodic law and a periodic table given by Mendeleev. In the latter part of the 18th century, Mendeleev made his periodic table. Scientists did not know about the internal structure of the atom back then.
What are S-block elements?
S-lock and P-block elements come under the category of representative elements. Elements in groups 1 and 2 are known as the s – block elements (elements with 1s2and 2s2 outermost configuration). Group 13-17 are known as the p-block elements (outermost configuration varies from ns2np1 to ns2np5).
What is periodic trend?
Periodic trends are common patterns in the periodic table showing us the various aspects of an element such as electronegativity, atomic radius, or ionizing power. The periodic law tells us that when grouped by atomic number, certain properties of elements occur periodically.
What is the long form of the periodic table?
The present form of a periodic table that is widely used across the globe is the long form of the periodic table. In this form of a periodic table, the horizontal rows are called periods and the vertical columns are known as the groups. Groups consist of elements that have similar outer shell electronic configuration in their atoms.
What is the atomic number of an atom?
The atomic number is equal to the number of electrons or protons in a neutral atom. After knowing the fundamental unit of elements, scientists now had a clear idea about quantum numbers and electronic configuration of elements in the periodic table. After knowing the periodic law, chemists noticed that there is an analogy between the 94 naturally occurring chemical elements. This analogy made people more curious about the chemistry of these elements. Scientists made various artificial elements. A new periodic table was developed based on the modern periodic law by modifying the Mendeleev’s periodic table.
What did scientists not know about the internal structure of the atom back then?
The development of various atomic models and advances in quantum theory revealed that the atomic number is the most basic property of a chemical element. This led to the modification of Mendeleev’s periodic law, which is today called as modern periodic law.
How many ways can you classify elements in the periodic table?
Classification of the elements in the periodic table can be done in four ways on the basis of their electronic configurations:
How did Mendeleev organize the periodic table?
Several chemists tried to make tables of the elements before Mendeleev’s periodic table, usually ordering elements by atomic mass (the concept of atomic number did not yet exist). Mendeleev also started by ordering the elements based on atomic mass, but he used the periodic repetition of elemental properties to do two very important things: he switched some elements close in mass so that they followed periodicity, resulting in a table organized by atomic number
What is the definition of periodic law?
Modern periodic law states that “ The properties of elements are the periodic functions of their atomic number”.
What is the difference between the periodic table and the modern periodic law?
As per the modern periodic law all the elements (some exceptions of are arranged as per their atomic mass whereas the former periodic table was categorized according to the atomic masses of the elements.
What is the law of periodicity?
Modern periodic law states that properties of elements are periodic functions of their atomic numbers.
What is the modern periodic table?
Modern Periodic Table is the arrangement of all known elements in the increasing order of their ATOMIC NUMBERS .
What is the long form of the periodic table called?
The long form of periodic table consist of vertical columns called groups and horizontal rows called periods.
What is the conclusion that has been reached comparing and contrasting the physical and chemical properties of all the elements?
Periodicity is the conclusion that has been reached comparing and contrasting the physical and chemical properties of all the elements. A pattern in their behaviour can be seen.
