How do we determine the mass number of an element?
What is Mass Number?
- The number of protons and neutrons combined to give us the mass number of an atom.
- It is represented using the letter ‘A.’
- As both protons and neutrons are present in the nucleus of an atom, they are together called nucleons.
- For example, an atom of carbon has 6 protons and 6 neutrons. ...
How do you calculate the mass of an element?
Part 3 Part 3 of 3: Measuring Mass with a Balance
- Use a triple-beam balance. The balance is a device widely used to calculate an object's mass. ...
- Move the three sliders to their leftmost positions. You want to do this maneuver when the pan is empty.
- Move the sliding beams one at a time. First, move the 100-gram slider along the beam to the right first.
- Calculate the mass. ...
How do you convert mass into moles of an element?
Moles to Grams Conversion Problem
- Problem. Determine the mass in grams of 3.60 mol of H 2 SO 4 .
- Solution. First, look up the atomic masses for hydrogen, sulfur, and oxygen from the periodic table. The atomic mass is 1.008 for H, 32.06 for S, and 16.00 for O.
- Answer. There are 353 grams of H 2 SO 4 in 3.60 moles of H 2 SO 4 .
What does the molar mass of an element represent?
What does the molar mass of an element represent? the value of the element’s atomic weight in units of grams per mole the mass of 1 mole of the element the mass of 1000 atoms of the element both choices a and b
What is Molar Mass?
How to find the molar mass of each element?
What is the mass of all the atoms of a molecule in grams per mole?
What is the mass of 0.5 moles of salt?
What is the unit used to measure molar mass?
Why is molar mass important?
How many molar masses does acetic acid have?
See more
About this website
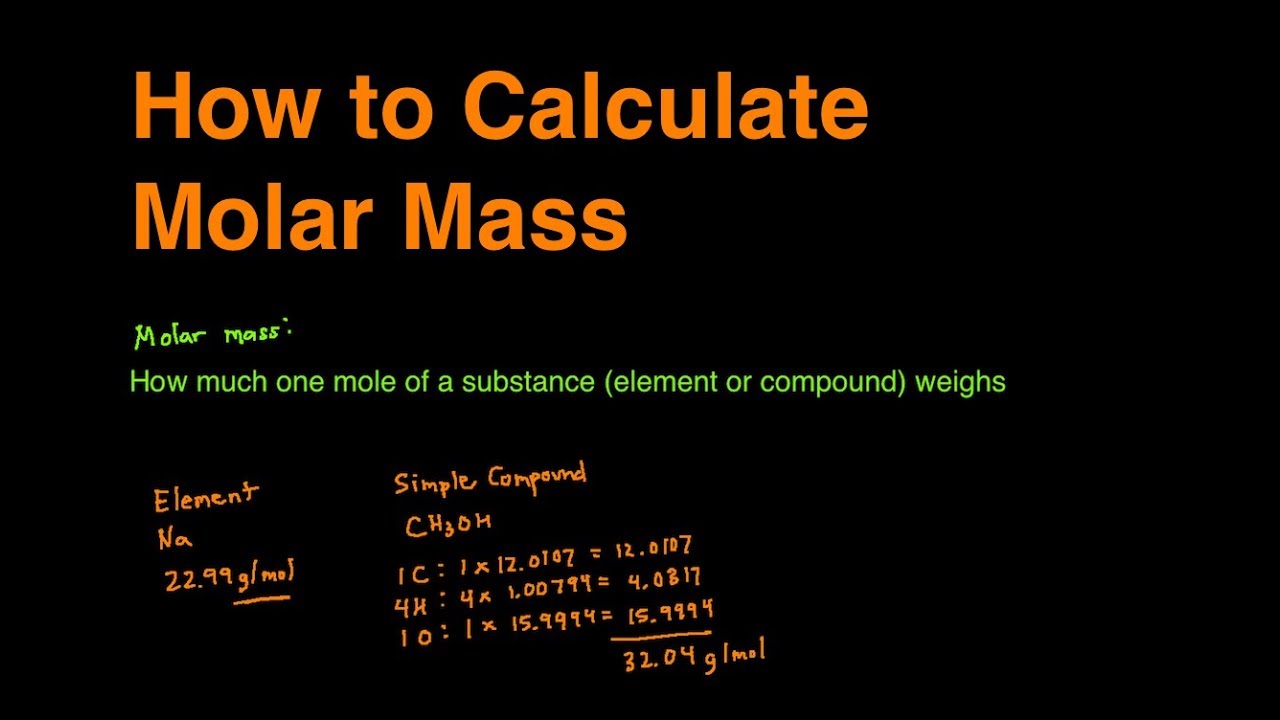
How do you find the molar mass of an element?
1:3011:20How To Calculate The Molar Mass of a Compound - Quick & Easy!YouTubeStart of suggested clipEnd of suggested clipSo the atomic mass of oxygen is 16 if you multiply by 3 that's 48 so the molar mass of ozone o3 it'sMoreSo the atomic mass of oxygen is 16 if you multiply by 3 that's 48 so the molar mass of ozone o3 it's 48 grams per mole.
What is meant by molar mass of an element?
Molar mass is defined as the mass in grams of one mole of a substance. The units of molar mass are grams per mole, abbreviated as g/mol. The mass of a single isotope of any given element (the isotopic atomic mass) is a value relating the mass of that isotope to the mass of the isotope carbon-12 (
What is the molar mass of all elements?
Molar Mass Click to see citationsElementRounded ( for regular calculations )Aluminum26.98 g/molAntimony121.8 g/molArgon39.95 g/mol81 more rows
What is molar mass of an atom?
Molar mass = mass/mole = g/mol The mass of one atom of carbon-12 the atomic mass of carbon-12 is exactly 12 atomic mass units. The mass of one mole of carbon-12 atoms is exactly 12 grams; its molar mass is exactly 12 grams per mole.
Is molar mass and atomic mass same?
Molar masses of the elements are the same numeric value as the masses of a single atom in atomic mass units but in units of grams instead. Molar masses of compounds are calculated by adding the molar masses of their atoms.
How do you find the moles of an element?
The unit is denoted by mol.The formula for the number of moles formula is expressed as.Given.Number of moles formula is.Number of moles = Mass of substance / Mass of one mole.Number of moles = 95 / 86.94.
What is the mass number of element?
1 Answer. Sana T. Mass number: the total number of neutrons and protons in an atom..
What is atomic mass of all elements?
For example, a normal carbon atom with six neutrons and six protons is denoted as carbon-12. It has an atomic mass equal to 12 amu....Atomic Number of Elements from 1 to 30.Atomic NumberElementAtomic Mass4Beryllium9.01225Boron10.816Carbon12.0117Nitrogen14.00726 more rows
What is the molar mass of c02?
44.01 g/molCarbon dioxide / Molar mass
What is the molar mass of h2o?
18.01528 g/molWater / Molar mass
What is the molar mass of the gas?
The molar mass of a gas can be derived from the ideal gas law, PV=nRT , by using the definition of molar mass to replace n , the number of moles. Here's an example of how this would look in a problem: An unknown gas has a mass of 153 g and occupies 15.0 L at a temperature of 300.0 K and a pressure of 2.00 atm.
Molar mass : table of 465 common chemical compounds
The molar mass of a substance, also often called molecular mass or molecular weight (although the definitions are not strictly identical, but it is only sensitive in very defined areas), is the weight of a defined amount of molecules of the substance (a mole) and is expressed in g/mol.
Molar mass - Wikipedia
The molecular mass (m) is the mass of a given molecule: it is usually measured in daltons (Da or u).Different molecules of the same compound may have different molecular masses because they contain different isotopes of an element. This is distinct but related to the molar mass, which is a measure of the average molecular mass of all the molecules in a sample and is usually the more ...
How to find the molar mass of a chemical?
To find the molar mass, find the atomic mass of all the components of a chemical. You can either memorize it, or find all of the atomic masses located on the periodic table of elements. In this case, hydrogen has an atomic mass of 1, and oxygen has an atomic mass of 16. The equation is therefore: 1 (2) + 16 (1) = 18.
How to find molar mass?
Molar mass is the mass (in grams) of one mole of a substance. Using the atomic mass of an element and multiplying it by the conversion factor grams per mole (g/mol), you can calculate the molar mass of that element.
How many grams are in a mole of hydrogen?
This converts atomic units to grams per mole, making the molar mass of hydrogen 1.007 grams per mole, of carbon 12.0107 grams per mole, of oxygen 15.9994 grams per mole, and of chlorine 35.453 grams per mole. Some elements are only found in molecules of 2 atoms or more.
What is the mass of one mole of glucose?
For glucose, the molar mass is 72.0642 + 12.084 + 95.9964 = 180.1446 g/mol. 180.14 grams is the mass of one mole of glucose.
How to find the relative amount of each element in a compound?
Calculate the molar mass of each element in the compound. Multiply the element's atomic mass by the number of atoms of that element in the compound. This will give you the relative amount that each element contributes to the compound.
How many atoms are in a mole?
A mole is defined as the number of carbon atoms in 12 grams of the isotope carbon-12, which is roughly 6.022 x 10 23 atoms. This number is called Avogadro's number or Avogadro's constant.
What are the relative atomic masses of glucose?
The relative atomic masses of the elements in glucose are: carbon, 12.0107 g/mol; hydrogen, 1.007 g/mol; and oxygen, 15.9994 g/mol. Calculate the molar mass of each element in the compound. Multiply the element's atomic mass by the number of atoms of that element in the compound.
What is Molar Mass?
Substances take up space and have mass. Molecules, which make up substances, often need to be measured in experiments, and it is important that these measurements are accurate. But, how can we measure something so small in an accurate way? How do we normally measure molecules? In the science laboratory, we use a tool called an analytical balance to measure in grams.
How to find the molar mass of each element?
Now we can find the molar mass of each element. For carbon, we multiply its molar mass of 12.0107 grams per mol by two because we have two carbon atoms. This equals 24.0214 grams per mole. For hydrogen, we multiply the molar mass of 1.00794 by 4. This equals 4.03176 grams per mol. Finally, for oxygen, we multiply the molar mass of 15.9994 by 2. This equals 31.9988 grams per mol.
What is the mass of all the atoms of a molecule in grams per mole?
Molar mass is the mass of all the atoms of a molecule in grams per mole.
What is the mass of 0.5 moles of salt?
When we multiply these two together, we find that the mass of 0.5 moles of salt is 29.22 grams.
What is the unit used to measure molar mass?
The unit used to measure is grams per mole. {"error":true,"iframe":true}. You must c C reate an account to continue watching. Register to view this lesson.
Why is molar mass important?
The molar mass is also an important conversion factor to convert moles to grams. To do this, we multiply the number of moles of the substance to the molar mass. This conversion factor is important when quantifying the weight in grams in scientific experiments. Quick Notes.
How many molar masses does acetic acid have?
Find the molar mass of acetic acid. There are three elements - carbon, hydrogen and oxygen - so find their atomic masses in the periodic table: 12.0107 grams per mol for carbon, 1.00794 grams per mol for hydrogen, and 15.9994 grams per mol for oxygen.
