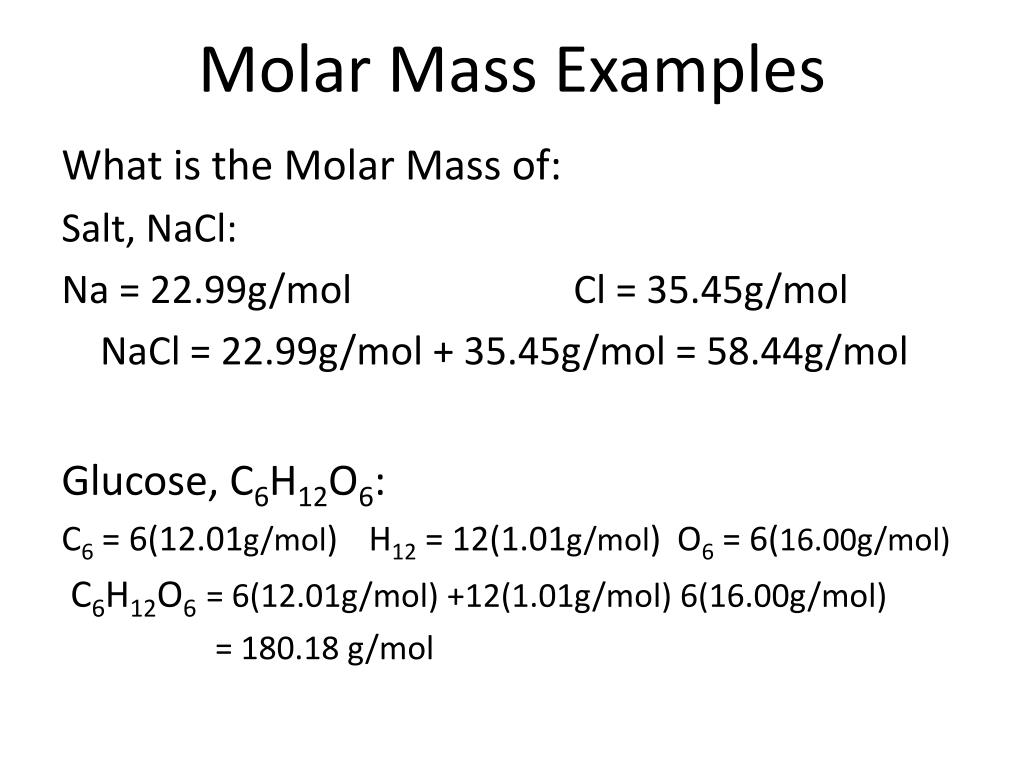
How do you determine molar mass?
- Determine the molar concentration of the unknown in the solution from the observed osmotic pressure.
- Determine the moles of unknown (the solute) from the molarity of the solution and the volume (in liters) of the solution.
- Determine the molar mass from the mass of the unknown and the number of moles of unknown. Top
What is the formula to find molar mass?
Molar Mass Formula
- Molar Mass Formula. The molar mass of a compound is the mass of a given substance divided by the quantity of substance therein sample.
- Calculation of Molar Mass. The molar mass of a compound is often calculated by adding the quality atomic masses (in g/mol) of the constituent atoms.
- Solved Examples for Molar Mass Formula. ...
How do you calculate mol weight?
You calculate molecular weight by taking the atomic weight and multiplying it by how many of that element you have in your molecule, and then to add up each ...
How do you calculate the mass of a molecule?
Method 2 Method 2 of 2: Calculating the Molar Mass of a Compound
- Find the chemical formula for the compound. This is the number of atoms in each element that makes up the compound.
- Find the relative atomic mass of each element in the compound. Using the periodic table, locate the relative atomic mass for each element.
- Calculate the molar mass of each element in the compound. ...

How do you find the mass of FeCl2?
0:151:07Molar Mass / Molecular Weight of FeCl2: Iron (II) chloride - YouTubeYouTubeStart of suggested clipEnd of suggested clipWe find the molar mass for fecl2. To be 126.75 and the units are grams per mole that means if weMoreWe find the molar mass for fecl2. To be 126.75 and the units are grams per mole that means if we have one mole of fecl2. This iron ii chloride.
What is the MR FeCl2?
Iron(II) chloride, also known as ferrous chloride, is the chemical compound of formula FeCl2.
What is the percent by mass of chlorine in FeCl2?
The atomic mass of iron (III) chloride is 162.20 g. The mass percent of chlorine atom can be calculated as shown below. Substitute the respective values in the above equation. Therefore, the percent of chlorine in iron (III) chloride is 65.5%.
How many grams are in FeCl2?
The molar mass of ferrous chloride is 126.75 g/mol. Where, n is the number of moles of solute. Hence, the mass of ferrous chloride is 23.8 g.
What FeCl2 called?
CHEBI:30812 - iron dichloride Iron(II) chloride, also known as ferrous chloride, is the chemical compound of formula FeCl2.
What is the formula for FeCl2?
FeCl₂Iron(II) chloride / Formula
How do I calculate molar mass?
Multiply the element's atomic mass by the number of atoms of that element in the compound. This will give you the relative amount that each element contributes to the compound. For hydrogen chloride, HCl, the molar mass of each element is 1.007 grams per mole for hydrogen and 35.453 grams per mole for chlorine.
How do you find the percent by mass of an element?
To calculate the mass percent of an element in a compound, we divide the mass of the element in 1 mole of the compound by the compound's molar mass and multiply the result by 100.
What is the percent of Fe in FeCl3?
34.3%FeCl3 has one iron atom and 3 Cl atoms. The entire compound has a molar mass of roughly 162 g/mol. The Fe roughly has a percent composition of 34.3%.
How do you find moles from grams and molar mass?
To correctly estimate the number of moles, n , of a substance of a specific mass, m , (in grams), you need to follow the grams to moles formula: n = m / M , where, M is the molar mass of this material.
How do you convert from moles to grams?
Multiply the given number of moles (2.50 mol) by the molar mass (122.548 g/mol) to get the grams.
What is the molar mass of iron?
55.845 uIron / Atomic mass
What FeCl3 called?
Ferric Chloride | FeCl3 - PubChem.
Is fecl2 a salt?
Iron (II) chloride crystals. This contains iron in its 2+ oxidation state, meaning it is bonded to two chloride ions, with the formula FeCl2. The salt is coloured, as are the salts of many transition metals.
Is fecl2 ionic or covalent?
FeCl2 is ionic compound but it has some covelent character determind by the Fajan's rule which state that small size positive charge with high positive charge densities polarizes a large sized negatively charge species and pull its electronic cloud towards itself that protects compound to be completely an ionic ...
What is iron formula?
Molecular Formula. Fe. Synonyms. 7439-89-6.