What is the molecular weight of tin (II) chloride?
›› Tin(II) Chloride molecular weight. Molar mass of SnCl2 = 189.616 g/mol. Convert grams Tin(II) Chloride to moles or moles Tin(II) Chloride to grams. Molecular weight calculation:
What is the molar mass of SnCl2 in grams?
Molar mass of SnCl2 = 189.616 g/mol. Convert grams Tin(II) Chloride to moles or moles Tin(II) Chloride to grams.
How is tin (II) chloride used in textiles?
An electric potential is applied, and tin metal is formed at the cathode via electrolysis. Tin(II) chloride is used as a mordant in textile dyeing because it gives brighter colours with some dyes e.g. cochineal. This mordant has also been used alone to increase the weight of silk.
What is the best way to dissolve tin (II) chloride?
Therefore, if clear solutions of tin(II) chloride are to be used, it must be dissolved in hydrochloric acid (typically of the same or greater molarity as the stannous chloride) to maintain the equilibrium towards the left-hand side (using Le Chatelier's principle).
What is the molar mass of tin IV chlorate?
The molecular weight of Tin (IV) chloride is 260.5 g/mol.
How do you make SnCl2?
Anhydrous SnCl2 is prepared by the action of dry hydrogen chloride gas on tin metal. The dihydrate is made by a similar reaction, using hydrochloric acid: Sn( s) + 2 HCl( aq) → SnCl2(aq) + H2(g) The water is then carefully evaporated from the acidic solution to produce crystals of SnCl2·2H2O.
Is SnCl2 an ionic compound?
SnCl2 is ionic but SnCl4 is covalent.
Why is SnCl2 covalent?
Is Tin (II) chloride ionic or covalent? In SnCl2, Sn has +2 oxidation state. According to Fajan's rule, the central metal with more oxidation number will be considered to be more covalent.
What is the name of SnCl2?
stannous chlorideTin(II) chloride, also known as stannous chloride, is a white crystalline solid with the formula SnCl2. It forms a stable dihydrate, but aqueous solutions tend to undergo hydrolysis, particularly if hot. SnCl2 is widely used as a reducing agent (in acid solution), and in electrolytic baths for tin-plating.
What is the role of SnCl2?
Stannous chloride (SnCl2) is widely used in daily human life to conserve soft drinks, in food manufacturing and biocidal preparations. It had genotoxicity, immunotoxicity, neurotoxicity and oxidative stress.
Why SnCl2 is ionic?
But for `SnCl_2`,Sn is in +2 oxidation state i.e. cation is bigger in size and carry less positive charge resulting low polarising power of the cation. Thus `SnCl_2` is ionic in nature.
Why is SnCl2 more ionic?
SnCl2 is more ionic than SnCl4 : Reason : According to Fajan rule compound with more charge has higher tendency to polarise the surrounding atom which tends to introduce a covalent character in molecule.
What is the molecular geometry of SnCl2?
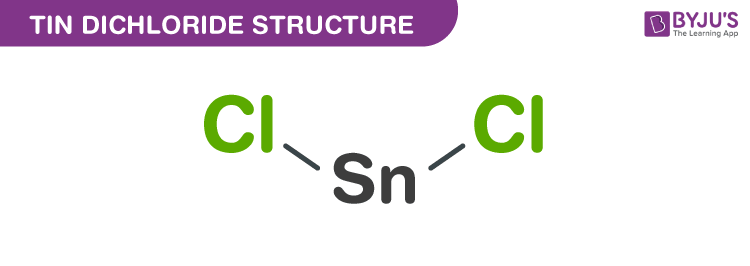
trigonal pyramidal shapeThe structure of SnCl2 is a trigonal pyramidal shape ( V shape) due to the presence of lone pair of electrons based on VSPER theory.
Is SnCl2 a solid?
SnCl2 is a solid while SnCl4 is a liquid.
Which is more covalent SnCl2 or SnCl4?
(ii) SnCl4 is more covalent than SnCl2.
Which is more stable SnCl2 or SnCl4?
Tin contains two electrons in d-orbital and hence show two oxidation state of +2 and +4 due to inert pair effect. as we move down the group (I.e. from Ge to Pb) stability of +2 oxidation state increases while that of +4 oxidation state decreases. Therefore, SnCl2 is more formed than SnCl4.
What is the formula of tin chloride?
SnCl₂Tin(II) chloride / Formula
How do you make a mercuric chloride solution?
Mercuric Chloride Shake 1.0 g with 10 mL of ethyl alcohol for 5 min, and filter. To 5 mL of the filtrate, add 0.10 mL of hydrochloric acid and 5 mL of freshly prepared hydrogen sulfide water.
What is the solution of tin (II) chloride?
Solutions of tin (II) chloride can also serve simply as a source of Sn 2+ ions, which can form other tin (II) compounds via precipitation reactions. For example, reaction with sodium sulfide produces the brown/black tin (II) sulfide : SnCl 2 (aq) + Na 2 S (aq) → SnS (s) + 2 NaCl (aq)
What is tin chloride used for?
A solution of tin (II) chloride containing a little hydrochloric acid is used for the tin-plating of steel, in order to make tin cans. An electric potential is applied, and tin metal is formed at the cathode via electrolysis . Tin (II) chloride is used as a mordant in textile dyeing because it gives brighter colours with some dyes e.g. cochineal.
What is the E number for stannous chloride?
Stannous chloride is also added as a food additive with E number E512 to some canned and bottled foods, where it serves as a color-retention agent and antioxidant .
What is the name of the compound that is a white crystalline solid?
Chemical compound. Tin (II) chloride, also known as stannous chloride, is a white crystalline solid with the formula Sn Cl 2. It forms a stable dihydrate, but aqueous solutions tend to undergo hydrolysis, particularly if hot.
What is tin used for?
Tin (II) chloride also finds wide use as a reducing agent.
Why is tin used as a mordant?
Tin (II) chloride is used as a mordant in textile dyeing because it gives brighter colours with some dyes e.g. cochineal. This mordant has also been used alone to increase the weight of silk.
Does tin chloride reduce silver?
This can be prevented by storing the solution over lumps of tin metal. There are many such cases where tin (II) chloride acts as a reducing agent, reducing silver and gold salts to the metal, and iron (III) salts to iron (II), for example: It also reduces copper (II) to copper (I).
How to find the molar mass of a molecule?
Take a standard chemistry formula for a molecule, split it up into the component atoms, and look up the molar weight of each atom. Add the weight of the atoms in the molecule and you have the molar mass for the molecule.
How Does The Molar Mass Calculator Work?
We present the results in a table at the bottom of the molar mass calculator - it will show the count of atoms, the atomic weight of each element, and the molecular weight for the molecule. It solves for total mass of a molecular formula (average molecular weight).
Computing molar mass (molar weight)
To calculate molar mass of a chemical compound enter its formula and click 'Compute'. In chemical formula you may use:
Computing molecular weight (molecular mass)
To calculate molecular weight of a chemical compound enter it's formula, specify its isotope mass number after each element in square brackets.
Definitions of molecular mass, molecular weight, molar mass and molar weight
Molecular mass ( molecular weight) is the mass of one molecule of a substance and is expressed in the unified atomic mass units (u). (1 u is equal to 1/12 the mass of one atom of carbon-12)
How to find the molar mass of a molecule?
Take a standard chemistry formula for a molecule, split it up into the component atoms, and look up the molar weight of each atom. Add the weight of the atoms in the molecule and you have the molar mass for the molecule.
How Does The Molar Mass Calculator Work?
We present the results in a table at the bottom of the molar mass calculator - it will show the count of atoms, the atomic weight of each element, and the molecular weight for the molecule. It solves for total mass of a molecular formula (average molecular weight).
Overview
Chemical properties
Tin(II) chloride can dissolve in less than its own mass of water without apparent decomposition, but as the solution is diluted, hydrolysis occurs to form an insoluble basic salt:
SnCl2 (aq) + H2O (l) ⇌ Sn(OH)Cl (s) + HCl (aq)
Therefore, if clear solutions of tin(II) chloride are to be used, it must be dissolved in hydrochloric acid (typically of the same or greater molarity as the stannous chloride) to maintain the equilibrium towards …
Chemical structure
SnCl2 has a lone pair of electrons, such that the molecule in the gas phase is bent. In the solid state, crystalline SnCl2 forms chains linked via chloride bridges as shown. The dihydrate is also three-coordinate, with one water coordinated on to the tin, and a second water coordinated to the first. The main part of the molecule stacks into double layers in the crystal lattice, with the "second" wate…
Preparation
Anhydrous SnCl2 is prepared by the action of dry hydrogen chloride gas on tin metal. The dihydrate is made by a similar reaction, using hydrochloric acid:
Sn (s) + 2 HCl (aq) → SnCl2 (aq) + H2 (g)
The water then carefully evaporated from the acidic solution to produce crystals of SnCl2·2H2O. This dihydrate can be dehydrated to anhydrous using acetic anhydride.
Uses
A solution of tin(II) chloride containing a little hydrochloric acid is used for the tin-plating of steel, in order to make tin cans. An electric potential is applied, and tin metal is formed at the cathode via electrolysis.
Tin(II) chloride is used as a mordant in textile dyeing because it gives brighter colours with some dyes e.g. cochineal. This mordant has also been used alon…
Notes
• N. N. Greenwood, A. Earnshaw, Chemistry of the Elements, 2nd ed., Butterworth-Heinemann, Oxford, UK, 1997.
• Handbook of Chemistry and Physics, 71st edition, CRC Press, Ann Arbor, Michigan, 1990.
• The Merck Index, 7th edition, Merck & Co, Rahway, New Jersey, USA, 1960.