
What is CS2 used for in real life?
Carbon disulfide, also spelled as carbon disulphide, is a neurotoxic colorless volatile liquid with the formula CS 2.The compound is used frequently as a building block in organic chemistry as well as an industrial and chemical non-polar solvent.It has an "ether-like" odor, but commercial samples are typically contaminated with foul-smelling impurities.
Is CS2 a polar molecule?
Similarly, the CS2 molecule is non-polar because of following reason, There exists a small difference between the electronegativity of carbon and sulfur atoms that makes the C-S a slightly polar bond. Both C-S bonds have dipoles in opposite directions as a result it cancels each other and net dipole turns out to be zero.
Is CS2 ionic or molecular compound?
The compound is commonly used in organic chemistry as a building block, as well as a non-polar industrial and chemical solvent. Is CS2 ionic or molecular? Although it consist of carbon and sulphide ions, It is not an ionic compound because of its weak and insignificant polarity. I would rather call it a covalent compound.
What is the name for the formula CS2?
Carbon Disulfide - CS2
- CS 2 Uses (Carbon Disulfide)
- Carbon Disulfide Reactions
- Health Hazards
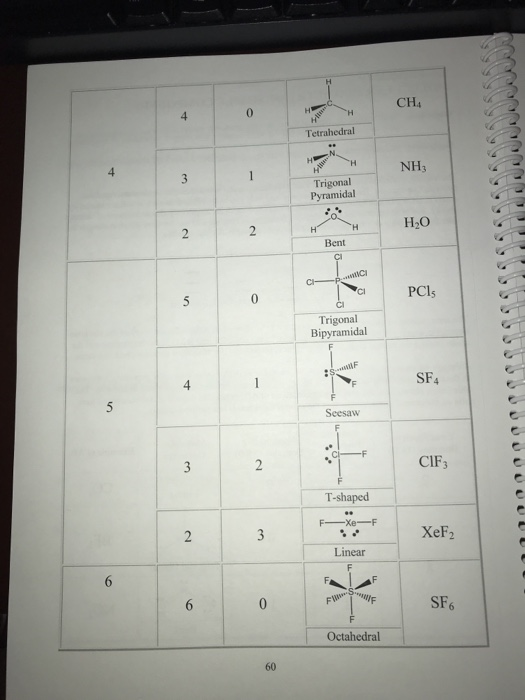
Is CS2 linear or tetrahedral?
linearThus, the molecular geometry of carbon disulfide is linear.
What type of bond is CS2?
CS2 is a covalent molecule as both the carbon and sulfur atoms have nearly the same electronegativity values. The electronegativity difference among the carbon and sulfur atom is nearly 0.03, and thus the bond between them is hardly even polar.
What is the molecular geometry of H2S?
bent shapeThe molecular geometry of H2S is bent shape or V-shape.
Is CS2 linear structure?
CS2 is linear whereas H2S is non-linear.
Is CS2 polar covalent?
CS2 (Carbon disulfide) is nonpolar because of its symmetric (linear) shape. Although carbon and sulfur differ in their electronegativity and C-S bond is polar, the polarity of both opposite C-S bonds gets canceled by each other resulting in a nonpolar molecule.
What is the molecular geometry shape of the carbon disulfide molecule CS2?
LinearThe molecular geometry of CS2 is Linear. It has a linear geometry arrangement like S=C=S. CS2 has 2 electron domains, resulting in a linear electron domain geometry. In the CS2 lewis structure, there is a total of 4 lone pairs present.
Does CO2 have linear molecular geometry?
CO2 Molecular Geometry Due to these repulsive forces between the valence shell electron pairs, the CO2 molecule acquires a linear shape to keep the repulsion at the least. Hence CO2 has a linear molecular geometry with the bond angles of 180 degrees and symmetric distribution of electrons.
Is H2S a trigonal planar?
Using VSEPR theory we use the sigma bonds to establish the geometry: three bonding pairs and no lone pairs on the central atom = trigonal planar. H2S has a linear molecular geometry.
Does CO2 have a bent shape?
Carbon dioxide is linear, while sulphur dioxide is bent (V-shaped). In the carbon dioxide, the two double bonds try to get as far apart as possible, and so the molecule is linear. In sulphur dioxide, as well as the two double bonds, there is also a lone pair on the sulphur.
Which of the following is the geometry and polarity of the CS2 molecule?
It will use one s and one p orbitals to form the hybrids, and the remaining p-orbitals to form pi bonds with the two sulfur atoms. The molecular geometry will thus be linear, the basic AX2 model.
What is the prediction of the geometry and polarity of the CS2 molecule?
The correct answer is option B. In the case of CS2, the central atom is a carbon (C) which is surrounded by 4 valence electrons. The individual bond dipoles due to two C=S bonds are in opposite directions in CS2 hence they cancel each other making the molecule non-polar. The CS2 molecule has linear molecular geometry.
Which is the correct Lewis structure for CS2?
There are 16 valence electrons for the CS2 Lewis structure. Carbon is the least electronegative atom and goes in the center of this structure. The Lewis structure for CS2 requires you have double bonds between the Carbon (C) and Sulfur atoms in order to fill the octet of Carbon.
Is CS2 ionic or molecular compound?
Carbon disulfideCarbon disulfide / IUPAC ID
Is carbon disulfide covalent or ionic bond?
covalent bondsAnswer and Explanation: Carbon disulfide is a compound made up of two nonmetallic elements, carbon and sulfur. Being purely nonmetals, it can be considered as a _molecular compound_ where covalent bonds between the atoms are formed by sharing of electrons.
Is co2 ionic or covalent?
Answer: In carbon dioxide molecule four covalent bonds are present. One carbon atom is joined to two oxygen atoms by four covalent bond , one oxygen atom have two covalent bond each.
Is CS2 a single bond?
It is essential to know the type of bonding in the molecule to understand its hybridization. In CS2 molecule, two double bonds are formed consisting of eight valence electrons. Thus it takes up eight valence electrons out of 16 valence electrons.
What is CS2 in biology?
CS2 is an abbreviated form of Carbon Disulphide. This molecule has two Sulphur atoms and one Carbon atom. To understand the hybridization, molecular geometry and the polarity of this molecule it is essential to under its Lewis structure.
How many orbitals does CS2 have?
Steric Number of CS2 is 2; thus its hybridization has two hybrid orbitals making it an sp hybridization.
How many sigma bonds are there in CS2?
Here in the CS2 molecule, the number of sigma bonds on the central atom is two , and there are no lone pairs on the central atom as its octet is complete by sharing the valence electrons. Steric Number of CS2 is 2; thus its hybridization has two hybrid orbitals making it an sp hybridization.
What is the name of the hybridization of carbon and sulfur?
This hybridization is known as sp hybridization . These two hybrid orbitals form sigma bonds with Carbon. Remaining eight valence electrons are taken up by the two unused orbitals of p. These electrons form the pi bonds with sulfur and are shown as the lone pairs on the sulfur atoms.
How to find the steric number of a molecule?
The formula to find the steric number for any molecule is: Steric Number (SN) = No of sigma bonds on the central atom +No of pi lone pairs on the central atom.
How to understand CS2 hybridization?
For understanding the hybridization of CS2 molecule, there are two easy methods. 1. To understand the bond formation and its type. It is essential to know the type of bonding in the molecule to understand its hybridization. In CS2 molecule, two double bonds are formed consisting of eight valence electrons.
What is the bond angle of CS2?
As the hybridization of CS2 is sp hybridization, the Carbon atom is in center bonding with two sulfur atoms forms the bond angle of 180 degrees, making the molecular geometry of CS2 molecule linear.
How many valence electrons does sulfur have?
1. Carbon belongs to Group 4 of the periodic table. Therefore, the number of valence electrons in the Carbon atom =4. Sulfur (S) belonging to Group 6 has 6 valence electrons. CS2 has two S atoms, hence, the valence electrons in sulfur here are 6*2=12. Total valence electrons is CS2 = 12+4 = 16. 2.
What is Lewis structure?
Lewis Structure is one of the key terminologies to understand the chemical bonding of a molecule since it represents the molecular structure. It depends on the octet rule concept and is an extension of the electron dot diagram. Thus, to have a comprehensive idea about CS2 Lewis Structure, let us go through each step clearly and systematically.
What is CS2 in chemistry?
Carbon disulfide or CS2 is one of the very common molecules we come across while studying chemistry. If you have ever come across the formation of carbon tetrachloride, a well-known reaction while studying chlorine, you have heard about CS2. Now let us learn about this sulfide in a detailed manner.
How to draw the structure of a molecule?
The very first step towards drawing the structure of a molecule is to decipher the total number of valence electrons. (A valence electron is a name given to the outer shell electron of an atom that takes part in the creation of a chemical bond).
What is CS2 used for?
It can be used for the production of viscose rayon and cellophane.
Why are polar molecules neutral?
A polar molecule will result due to unequal sharing of electrons whereas non-polar ones are neutral.
What does the positive + sign mean?
A very important point to be noted here is the role of the signs ‘+’ and ‘-’. While the positive ‘+’ sign indicates the loss of electrons i.e loss of negative charge, the negative ‘-’ sign is to denote the gain of electrons. Step 2. The second step is based on finding the central atom of the molecule.

Lewis Structure of CS2
Hybridization of CS2
Molecular Geometry of CS2
- As the hybridization of CS2 is sp hybridization, the Carbon atom is in center bonding with two sulfur atoms forms the bond angle of 180 degrees, making the molecular geometry of CS2 molecule linear. The general formula for linear geometry is AX2, and thus CS2 shows linear geometry.
Polarity of CS2
Conclusion