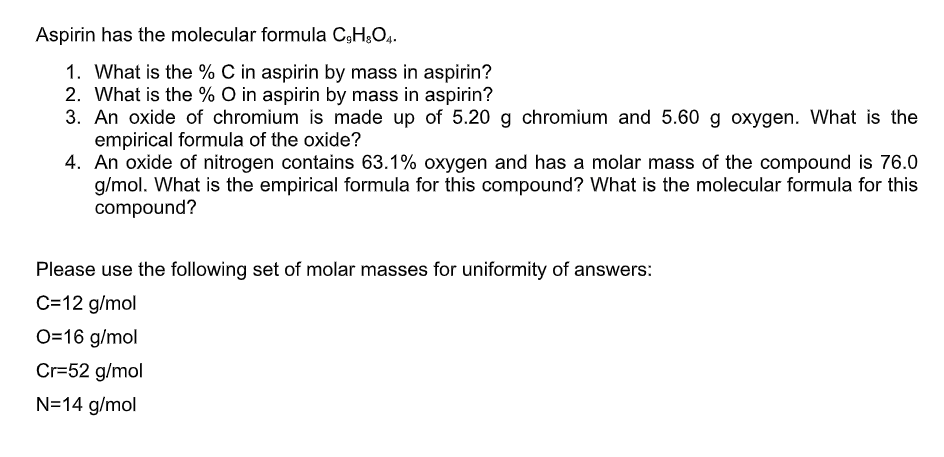
What is the molar mass of C9H8O4?
Molar mass of C9H8O4 = 180.15742 g/mol. This compound is also known as Aspirin. Convert grams C9H8O4 to moles or moles C9H8O4 to grams. Molecular weight calculation: 12.0107*9 + 1.00794*8 + 15.9994*4.
What is the molecular weight of aspirin in grams?
›› Aspirin molecular weight. Molar mass of C9H8O4 = 180.15742 g/mol. Convert grams Aspirin to moles or moles Aspirin to grams. Molecular weight calculation: 12.0107*9 + 1.00794*8 + 15.9994*4 ›› Percent composition by element
What are the properties of aspirin?
This agent exhibits analgesic, antipyretic, and anticoagulant properties. Also known as Aspirin, acetylsalicylic acid (ASA) is a commonly used drug for the treatment of pain and fever due to various causes.
What is the stability of aspirin in aqueous solution?
In aqueous solutions, aspirin is most stable at a pH of 2-3, less stable at a pH of 4-8, and least stable at a pH less than 2 or greater than 8. In a saturated aqueous solution at a pH of 5-7, aspirin is almost completely hydrolyzed within 1 week at 25 deg C. McEvoy, G.K. (ed.). American Hospital Formulary Service.
What is the molar mass of aspirin with the molecular formula of C9H8O4?
180⋅g/mol.
What is the molecular weight of aspirin?
180.158 g/molAspirin / Molar mass
What is the O in aspirin by mass in aspirin C9H8O4?
Likewise, the molecular mass of an aspirin molecule, C9H8O4, is the sum of the atomic masses of nine carbon atoms, eight hydrogen atoms, and four oxygen atoms, which amounts to 180.15 amu (Figure 3.3).
How many grams are in 1 mole of aspirin C9H8O4?
This means that one mole of aspirin will have a mass of 180.157 g .
What's C9H8O4?
Aspirin | C9H8O4 - PubChem.
What is the percent composition of aspirin C9H8O4?
Results for: C9H8O4ElementNumberPercet CompositionH84.47574619823572O435.5253195613511C959.9989342404132
What is the %C in aspirin by mass in aspirin?
60.0%Aspirin is made of H, O & C, and was analyzed to contain 60.0% carbon and 35.5% Oxygen.
How do you calculate MR of aspirin?
180.158 g/molAspirin / Molar mass
How do I calculate molar mass?
Molar mass = mass/mole = g/mol The mass of one mole of carbon-12 atoms is exactly 12 grams; its molar mass is exactly 12 grams per mole.
How many atoms are in 1 mole of C9H8O4?
MOLES OF ELEMENTS IN A FORMULA For example, in a molecule of aspirin, C9H8O4, there are 9 carbon atoms, 8 hydrogen atoms and 4 oxygen atoms.
How many moles of aspirin C9H8O4 contain 0.480 mol of O?
Solution for problem 4SC Chapter 6 Step-1Given:Moles of O = 0.480 mol O1 mol of C9H8O4 = 4 mol of OThus we can use the conversion factor, 0.480 mol of O = 0.480 mol of O = 0.12 mol of C9H8O4Thus 0.12 mol of aspirin, C9H8O4, contain 0.480 mole of O.
How do you calculate grams to moles?
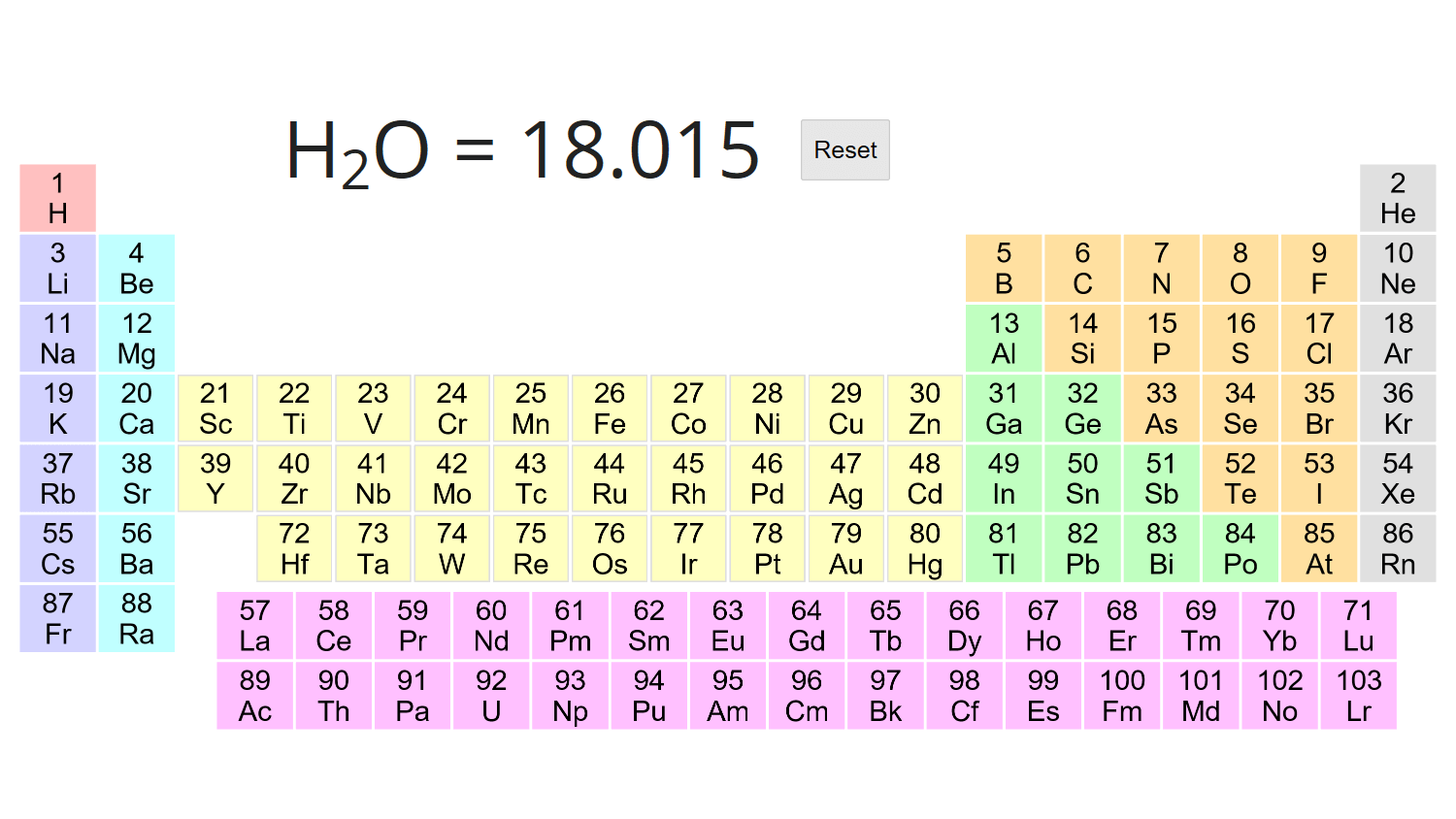
Divide the mass of the substance in grams by its molecular weight. This will give you the number of moles of that substance that are in the specified mass. For 12 g of water, (25 g)/(18.015 g/mol) = 0.666 moles.
What is the molecular formula of aspirin?
C₉H₈O₄Aspirin / Formula
What is the mass of aspirin in grams?
The mass of aspirin and filter paper was 3.159 grams. The mass of the filter paper was . 1300 grams. Thus, the calculated value of crude synthesized aspirin was 3.029 grams.
How many moles are in aspirin?
MOLES OF ELEMENTS IN A FORMULA For example, in a molecule of aspirin, C9H8O4, there are 9 carbon atoms, 8 hydrogen atoms and 4 oxygen atoms. compound. For example, one mole of aspirin contains 9 moles of carbon atoms, 8 moles of hydrogen atoms and 4 moles of oxygen atoms.
What is the density of aspirin in g mL?
1.35g/mLSpecificationsDensity1.35g/mLQuantity100gFormula Weight180.16Physical FormSolidChemical Name or MaterialAcetylsalicylic Acid2 more rows
How much aspirin is lethal?
The lethal dose of aspirin for an adult is probably in the region of 25 to 30 g but recovery has been achieved by appropriate treatment after the ingestion of twice or thrice this amount.
How many aspirin tablets should be stored?
Chewable aspirin tablets containing 81 mg of the drug should be stored in child-resistant containers holding not more than 36 tablets each in order to limit the potential toxicity associated with accidental ingestion in children. Aspirin suppositories should be stored at 2-15 °C.
How long does it take for aspirin to be detected in breast milk?
Acetylsalicylic acid and the acetylsalicylate ion were detected in the breast milk of nursing mothers within 1 hour of aspirin (acetylsalicylic acid) ingestion (1).
What is acetylsalicylic acid?
**Pain, fever, and inflammation** Acetylsalicylic acid (ASA), in the regular tablet form (immediate-release), is indicated to relieve pain, fever, and inflammation associated with many conditions, including the flu, the common cold, neck and back pain, dysmenorrhea, headache, tooth pain, sprains, fractures, myositis, neuralgia, synovitis, arthritis, bursitis, burns, and various injuries. It is also used for symptomatic pain relief after surgical and dental procedures [FDA label]. The _extra strength_ formulation of acetylsalicylic acid is also indicated for the management migraine pain with photophobia (sensitivity to light) and phonophobia (sensitivity to sound) [FDA label]. **Other indications** ASA is also indicated for various other purposes, due to its ability to inhibit platelet aggregation. These include: Reducing the risk of cardiovascular death in suspected cases of myocardial infarction (MI) [FDA label]. Reducing the risk of a first non-fatal myocardial infarction in patients, and for reducing the risk of morbidity and mortality in cases of unstable angina and in those who have had a prior myocardial infarction [FDA label]. For reducing the risk of transient ischemic attacks (TIA) and to prevent atherothrombotic cerebral infarction (in conjunction with other treatments) [FDA label]. For the prevention of thromboembolism after hip replacement surgery [FDA label]. For decreasing platelet to platelet adhesion following carotid endarterectomy, aiding in the prevention of transient ischemic attacks (TIA) [FDA label]. Used for patients undergoing hemodialysis with a silicone rubber arteriovenous cannula inserted to prevent thrombosis at the insertion site [FDA Label]. **Important note regarding use of the extended-release formulation [F4405]** In the setting of acute myocardial infarction, or before percutaneous interventions, the extended-release form of acetylsalicylic acid should not be used. Use immediate-release formulations in scenarios requiring rapid onset of action [Label, F4405]. The extended-release form is taken to decrease the incidence of mortality and myocardial infarction (MI) for individuals diagnosed with chronic coronary artery disease (CAD), including patients with previous myocardial infarction (MI) or unstable angina or with chronic stable angina. Additionally, the extended-release form is used to decrease the risk of death and recurrent episodes of stroke in patients with a history of stroke or TIA [F4405].
What is ASA in a blood test?
Acetylsalicylic acid (ASA) blocks prostaglandin synthesis. It is non-selective for COX-1 and COX-2 enzymes [A177241, A10989, A32682]. Inhibition of COX-1 results in the inhibition of platelet aggregation for about 7-10 days (average platelet lifespan). The acetyl group of acetylsalicylic acid binds with a serine residue of the cyclooxygenase-1 (COX-1) enzyme, leading to irreversible inhibition. This prevents the production of pain-causing prostaglandins. This process also stops the conversion of arachidonic acid to thromboxane A2 ( TXA2 ), which is a potent inducer of platelet aggregation [FDA label]. Platelet aggregation can result in clots and harmful venous and arterial thromboembolism, leading to conditions such as pulmonary embolism and stroke. It is important to note that there is 60% homology between the protein structures of COX-1 and COX-2. ASA binds to serine 516 residue on the active site of COX-2 in the same fashion as its binding to the serine 530 residue located on the active site of COX-1. The active site of COX-2 is, however, slightly larger than the active site of COX-1, so that arachidonic acid (which later becomes prostaglandins) manages to bypass the aspirin molecule inactivating COX-2 [A32682, A177256]. ASA, therefore, exerts more action on the COX-1 receptor rather than on the COX-2 receptor [A177268]. A higher dose of acetylsalicylic acid is required for COX-2 inhibition [A177325].
Is aspirin hydrolyzed?
Acetylsalicylic acid is hydrolyzed in the plasma to salicylic acid. Plasma concentrations of aspirin following after administration of the extended-release form are mostly undetectable 4-8 hours after ingestion of a single dose. Salicylic acid was measured at 24 hours following a single dose of extended-release acetylsalicylic acid [F4405]. Salicylate is mainly metabolized in the liver, although other tissues may also be involved in this process [FDA label]. The major metabolites of acetylsalicylic acid are salicylic acid, salicyluric acid, the ether or phenolic glucuronide and the ester or acyl glucuronide. A small portion is converted to gentisic acid and other hydroxybenzoic acids [FDA label].
When was the acetylsalicylic acid thioester patent issued?
Patent US4563443, issued March, 1981.
What is the formula for calculating molar mass?
If the formula used in calculating molar mass is the molecular formula , the formula weight computed is the molecular weight. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom ...
How to calculate weight in chemistry?
In chemistry, the formula weight is a quantity computed by multiplying the atomic weight (in atomic mass units) of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together. A common request on this site is to convert grams to moles.
What is formula weight?
The formula weight is simply the weight in atomic mass units of all the atoms in a given formula. Formula weights are especially useful in determining the relative weights of reagents and products in a chemical reaction. These relative weights computed from the chemical equation are sometimes called equation weights.
What is the molecular structure of aspirin?
The molecular structure image of aspirin is available in chemical structure page of aspirin, which provides the molecular geometry information, i.e., the spatial arrangement of atoms in aspirin and the chemical bonds that hold the atoms together.
How many atoms are in an aspirin molecule?
The aspirin molecule consists of 8 Hydrogen atom (s), 9 Carbon atom (s) and 4 Oxygen atom (s) - a total of 21 atom (s). The molecular weight of aspirin is determined by the sum of the atomic weights of each constituent element multiplied by the number of atoms, which is calculated to be:
Why is aspirin encoded?
It can provide a way to encode the molecular information of aspirin to facilitate the search for the compound information in databases and on the web.
What is the SDF file for aspirin?
The structure data file (SDF/MOL File) of aspirin is available for download in the SDF page of aspirin, which provides the information on atoms, bonds, connectivity and coordinates of aspirin. The aspirin structure data file can be imported to most of the chemistry-related software, providing three-dimensional visualization and further analysis.
Does aspirin have structural information?
In addition to the molecular weight information, the structural information of aspirin in a textual expression is available via InChi. The full standard InChI of aspirin is given below:
Can I use Inchikey to search for aspirin?
It may allow easier web searches for aspirin. The InChIKey, however, needs to be linked to the full InChI as well in order to get back to the original structure of the aspirin as the full standard InChI cannot be reconstructed from the InChIKey.
