
Is NABR an ionic or molecular bond?
NaBr is an ionic compound. Bromine has enough electronegativity that the electromagnetic force between the Br and the Na atoms is great enough that an electron transfers from the Na atom to the Br atom. So Br becomes negatively charged and Na becomes positively charged. How would you describe the following bond Na Br?
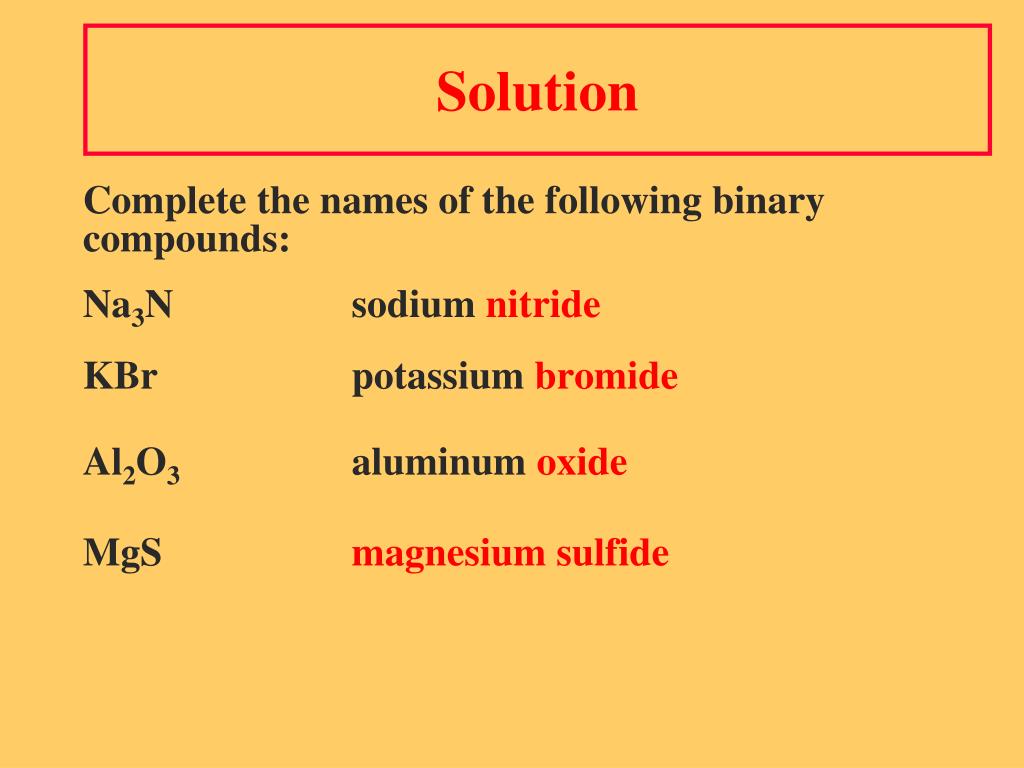
What are the rules for naming compounds?
What are the rules for naming ionic compounds quizlet?
- Balance charges (charges should equal zero)
- Cation is always written first (for name and formula)
- If the cation has more than one charge option, use the corresponding Roman numeral that matches. …
- Change the ending of the anion to -ide if it comes from the periodic table.
What is the name of the chemical NABR?
Uses of Sodium Bromide – NaBr
- Used as a sedative like other bromides.
- Used in oil and gas drilling industry is a principal consumer of sodium bromide.
- Used for its germicidal properties due to liberation of bromine.
- Used as an antiseptic, detergent, and as a reagent in pharmaceutical preparations.
What is the name of NaMnO4 compound?
Sodium permanganate | NaMnO4 or MnNaO4 | CID 23673458 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological ...

What is this compound made of NaBr?
Sodium bromideSodium bromide is a chemical compound. Its chemical formula is NaBr. It is made of sodium and bromide ions.
How do you name Sodium bromide?
0:001:13How to Write the Formula for NaBr (Sodium bromide) - YouTubeYouTubeStart of suggested clipEnd of suggested clipTo write the formula for sodium bromide we look on the periodic table and sodium that's n a that's aMoreTo write the formula for sodium bromide we look on the periodic table and sodium that's n a that's a metal. And the bromide ion that's the bromine. That's a non-metal.
Why is NaBr a compound?
Is sodium bromide covalent or ionic? Sodium bromide is an ionically bonded compound. The electronegativity of bromine is high enough and the electromagnetic force between the Br and the Na atoms is great enough that an electron is transferred from the Na atom to the Br atom.
Why is NaBr classified as compound?
9. Which statement explains why NaBr is classified as a compound? (2) Na and Br are both nonmetals. (3) NaBr is a solid at 298K and standard pressure.
Is bromine a sodium bromide?
Sodium bromide is an inorganic compound with the formula NaBr. It is a high-melting white, crystalline solid that resembles sodium chloride. It is a widely used source of the bromide ion and has many applications....Sodium bromide.NamesOther anionsSodium fluoride Sodium chloride Sodium iodide Sodium astatide53 more rows
How do you write sodium bromide formula?
NaBrSodium bromide / Formula
How do you name ionic compounds?
Ionic compounds are named by stating the cation first, followed by the anion. Positive and negative charges must balance. Some anions have multiple forms and are named accordingly with the use of roman numerals in parentheses.
What is the correct name for snbr4?
Tin(IV) bromide StannaneStannane, tetrabromo-PubChem CID24616StructureFind Similar StructuresChemical SafetyLaboratory Chemical Safety Summary (LCSS) DatasheetMolecular FormulaSnBr4 or Br4SnSynonymsTin(IV) bromide Stannane, tetrabromo- Tetrabromostannane Stannic bromide Tin tetrabromide More...2 more rows
What is the name of mgcl2?
Sodium bromide is a type of an ionic compound. The reason that this compound is ionic is that is is comprised of sodium that is a metal and also acts as a cation and bromine, which is a non-metal and acts as an anion. Hence, they form an ionic type of bond.
Why is sodium bromide NaBr?
The sodium bromide is an inorganic compound in its dry form a white crystalline powder with a salty and somewhat bitter taste. The chemical formula for sodium bromide in NaBr. … Other names – Bromide salt of sodium.
How do you name NaClO?
Sodium hypochlorite (commonly known in a dilute solution as bleach) is a chemical compound with the formula NaOCl or NaClO, comprising a sodium cation (Na+) and a hypochlorite anion (OCl−or ClO−).
Is cl2 NaBr NaCl Br2 balance?
How to Balance NaBr + Cl2 = NaCl + Br2 (Sodium Bromide + Chlorine gas)
What is NaBr cl2?
Word equation: Sodium Bromide + Chlorine gas → Sodium chloride + Bromine gas. Type of Chemical Reaction: For this reaction we have a single displacement reaction. Balancing Strategies: This is a typical single displacement reaction. The chlorine pushes the Br out from the NaBr.
Which test will identify NaBr?
Add concentrated sulfuric acid to aqueous NaCl and heat the solution HCl vapor is produced. Add concentrated sulfuric acid to aqueous NaBr and heat the solution. You will see brown color bromine vapor is produced.
How do you name sodium?
The name derives from the English soda and Latin sodanum for “headache remedy”. The symbol Na derives from the Latin natrium for “natron” (soda in English). Sodium was discovered in 1807 by the English chemist Humphry Davy from electrolysis of caustic soda (NaOH).
What is Sodium Bromide?
The sodium bromide is an inorganic compound in its dry form a white crystalline powder with a salty and somewhat bitter taste. The chemical formula for sodium bromide in NaBr. Its is a white crystal or white, granular powder having the odour of sulphur dioxide. It does not occur as natural solid due to its solubility, it is extracted from ocean water along with chlorides, iodides and halites. It possesses anticonvulsant properties of any bromide salt and one of the most common salts of hydrobromic acid.
Is sodium bromide ionically bonded?
Sodium bromide is an ionically bonded compound. The electronegativity of bromine is high enough and that the electromagnetic force between the Br and the Na atoms is great enough that an electron is transferred from the Na atom to the Br atom.
Is iodide a solid?
It does not occur as natural solid due to its solubility, it is extracted from ocean water along with chlorides, iodides and halites. It possesses anticonvulsant properties of any bromide salt and one of the most common salts of hydrobromic acid.
Is sodium bromide a sedative?
Used as a sedative like other bromides. Used in oil and gas drilling industry is a principal consumer of sodium bromide. Used for its germicidal properties due to liberation of bromine. Used as an antiseptic, detergent, and as a reagent in pharmaceutical preparations.
What is the reaction between sodium bromide and silver nitrate?
2. The reaction between Sodium bromide and silver nitrate solution results in the formation of silver bromide and sodium nitrate. The chemical equation is provided below.
What is the chemical name of NaBR?
The chemical name of nabr is sodium bromide . The sodium bromide structure is linear, and the sodium bromide structure is polar because the positive and negative radicals create a polarity where electrons get shared on the more electronegative radical that is the bromide liberal in sodium bromide.
What is the pH of sodium bromide?
Sodium Bromide. It is available in the color of white crystals, granules, or powder/white, cubic crystal options and it tastes a bit bad. Its other properties include Boiling Point that is 1390°C, Melting Point, that is 755°C, Density/Specific Gravity, that is of 3.21, pH ranges between 6.5-8.0 and solubility in alcohol is moderate). In water, it is 94.6 g/100 g water at 25°C. It is the physical property of sodium chloride. It is essential to know the ph. of the sodium bromide because it helps the doctors to know where and in which part of the body the use of sodium bromide is going to be. Now, we have to look after the ph. Factor so that no skin irritation in the living beings occurs and sodium chloride can be entirely safe for use.
How much sodium bromide is in water?
In water, it is 94.6 g/100 g water at 25°C. It is the physical property of sodium chloride. It is essential to know the ph. of the sodium bromide because it helps the doctors to know where and in which part of the body the use of sodium bromide is going to be.
Why is sodium bromide used in oil drilling?
In the oil and gas drilling industry, sodium bromide is a principal consumer because of its displacing properties, which can be used for the extraction of oils.
What chemical compounds are extracted from the ocean?
Some chemical compounds known as halides, chlorides and iodides also get extracted while withdrawing from the ocean water. Anticonvulsant properties of the bromide salts are exhibited within the other salts derived from the ocean water, and it’s the most common salt of the hydrobromic acid.
Which atom binds with the sodium ion?
Therefore, one hydrogen atom from the sulphuric acid binds with the sodium ion and brings down the oxidation state of the sodium ion.
Sodium Bromide
- What is sodium bromide? How can you define it? It is known as an inorganic compound having the formula NaBr and we widely use this inorganic compound as the source of bromide ions. Sodium bromide crystallises in the same manner as NaCl, NaF and NAI. And this anhydrous salt crystallises above 50.7 degrees celsius. Sodium bromide (NaBr) is produced b...
Sodium Bromide Structure – Nabr
- (Images will be uploaded soon) Sodium is a positively charged radical, which is very unstable because it is a highly reactive metal. Even to protect it from its reactivity, it is kept inside kerosene oil. So, this element reacts with the negatively charged bromide radical to form an ionic bond together to form a sodium bromide compound. Now, this ionic bond is mostly stable, and the Na…
Physical Properties of Sodium Bromide
- These were the physical properties of sodium bromide salt, which helps the chemists and druggists to make the use of sodium bromide salt. The physical properties of sodium bromide are also required by the chemical industries based on which they manufacture it for lab purposes. Chemical Properties of Sodium Bromide 1. The reaction between Sodium bromide and sulphuri…
Uses of Nabr
- Like any other bromides, it is used as a sedative for medical purposes. In many health problems, insomnia, sedatives are used to cure it. In the oil and gas drilling industry, sodium bromide is a principal consumer because of its displacing properties, which can be used for the extraction of oils. As the bromide ion is getting replaced by the ion of the more positively charged ions, sodiu…
Solved Example
- 1. What kind of salt is sodium bromide? Sodium bromide is a white, fully crystal-like substance. It is a compound made of sodium and bromide (a charged bromine atom). Its appearance resembles very closely regular table salt.