See more

Why is neon 20 the most abundant?
Stable isotopes of neon are produced in stars. Neon's most abundant isotope 20Ne (90.48%) is created by the nuclear fusion of carbon and carbon in the carbon-burning process of stellar nucleosynthesis.
What is the abundance of neon 22?
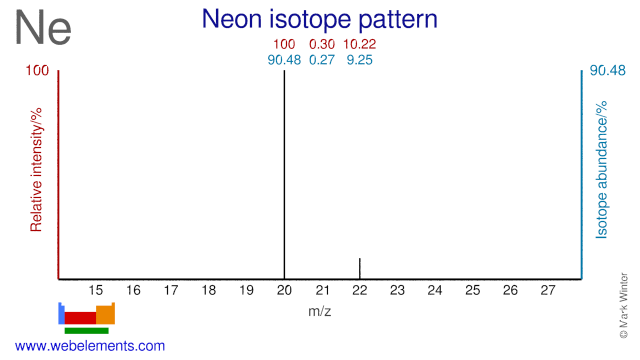
Isotopes of neonIsotopeDecayabundanceproduct20Ne90.48(3)%stable21Ne0.27(1)%stable22Ne9.25(3)%stable
What is the percent abundance of neon 21?
(0.27%)Neon has three isotopes: 20Ne (90.48%), 21Ne (0.27%) and 22Ne (9.25%).
How do you calculate percent abundance of neon?
There are two common isotopes of naturally occurring neon as indicated in the table below. (i) Using the information above, calculate the percent abundance of each isotope. Let x represent the natural abundance of Ne-20. ⇒ percent abundances are: Ne-20 = 90.5% Ne-22 = 9.5% One point is earned for the correct answer.
How do you find natural abundance?
How do you calculate the percentage of abundance based on mass? Calculate the average atomic mass using the atomic masses of each isotope and their percent abundances. Divide each percent abundance by 100 to convert it to decimal form. Multiply this value by the isotope's atomic mass.
What is the abundance of neon-20?
90.48%Page 2 of 3 At your desks: Neon has three naturally occurring isotopes: Ne-20 (90.48% abundance), Ne-21 (0.27% abundance), and Ne-22 (9.25% abundance).
How do you find the abundance of isotopes?
To calculate the percent abundance of each isotope in a sample of an element, chemists usually divide the number of atoms of a particular isotope by the total number of atoms of all isotopes of that element and then multiply the result by 100.
What are neon-20 and neon-22 called?
They are simply isotopes of each other. The Neon - 22 isotope has two extra neutrons in the nucleus than the Neon - 20 isotope. The atomic number and hence the proton number is the same in both.
What is the difference between neon-20 and neon-22?
The only difference between an atom of neon-20 and an atom of neon-22 is the number of neutrons in their nuclei. Neon-20 possesses 10 neutrons while neon-22 possesses 12 neutrons.
What is percent abundance?
What is Percent Abundance? Percent abundance is the percentage amount of all naturally occurring isotopes of an element. Isotopes are atoms of the same element that have identical atomic numbers but different mass numbers.
What Is percent abundance of an isotope?
The relative abundance of an isotope is the percentage of atoms with a specific atomic mass found in a naturally occurring sample of an element.
How do you find the abundance of 3 isotopes?
2:064:49Percent abundance of 3 isotopes with two unknowns - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo we'll say ninety-nine. Point six percent which. Remember it's just over a hundred right timesMoreSo we'll say ninety-nine. Point six percent which. Remember it's just over a hundred right times argon-40 argon-40 has a mass of actual mass of 39.96.
How many electrons does neon-22 have?
Answer and Explanation: The given isotope is neutral (uncharged). Therefore, it must contain 10 electrons to exactly balance its 10 protons.
How many protons does neon-22 have?
mass number and is given the symbol A. For neon, which has 10 protons, the mass numbers of the three different naturally occurring isotopes are 20, 21, and 22, corresponding to 10, 11, and 12 neutrons, respectively.
How many neutrons are there in neon-22?
12 neutronsNeon-20 possesses 10 neutrons while neon-22 possesses 12 neutrons. We can determine the number of neutrons based on the mass number of the isotope, the number listed after the element in its name, and the atomic number of the element.
What element is NE-22?
Neon4.3Related ElementElement NameNeonElement SymbolNeAtomic Number10
What is the Ne-22 isotope used for?
Element reactions. The three Neon isotopes are used for various purposes. Ne-22 is used for the production of the medical radioisotope Na-22. Ne-20 can be used for the production of F-18, although the route via O-18 is by far the most commonly used.
When was Mason in multinuclear NMR?
J. Mason in Multinuclear NMR, Plenum Press, New York, USA, 1987. Where given, data for certain radioactive nuclei are from this reference.
What is the abbreviation for natural abundance?
Natural abundance is the measure of the average amount of a given isotope naturally occurring on Earth. The abbreviation for natural abundance is NA. The atomic weight listed for each element on the periodic table is the natural abundance on Earth.
Why is the natural abundance of an element a global mean?
The natural abundance is a global mean, so if you sample an element at one location, you won't get exactly the average ratio of elements. Why is this so? Scientists believe the chemical composition of the solar system was isotopically homogeneous during its formation, but that deviations began to occur when fusion started in the Sun. Also, radioactive decay leads to differences in isotope ratios. This is because decay is a random process.
How many isotopes of boron are there?
There are two natural isotopes of boron: 10 B and 11 B. The natural abundance is 19.9% of 10 B and 80.1% of 11 B. Put another way, if you took a 100-gram sample of boron from anywhere on the planet, you could expect 19.9 grams to consist of boron-10 and 80.1 grams to consist of boron-11.