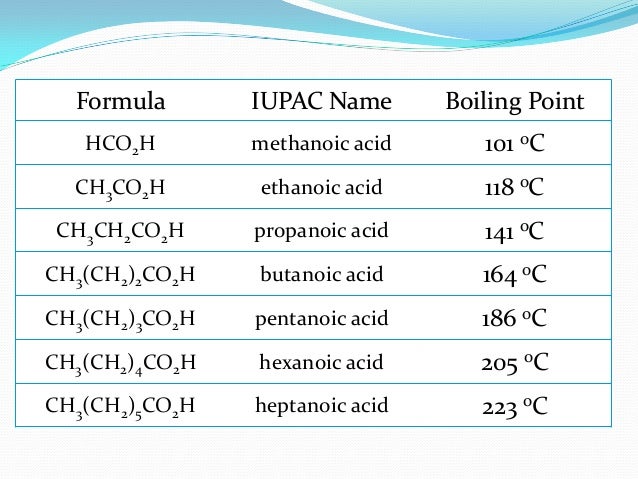
How do you calculate a normal boiling point?
To find the normal boiling point of a liquid, a horizontal line is drawn from the left at a pressure equal to standard pressure. At whatever temperature that line intersects the vapor pressure curve of a liquid is the boiling point of that liquid.
When does the normal boiling point occur?
The boiling point is defined as the temperature when the vapor pressure of the liquid is equal to the atmospheric pressure, which generally is constant unless you're changing the pressure intentionally. So from this point on we know that boiling point is constant if the atmospheric pressure is constant.
Which has the lowest normal boiling point?
Which Liquids have high boiling point?
- Acetone 56.0 ∘C .
- Ethanol 78.5 ∘C .
- Peanut oil 230 ∘C .
- Glycerol 290.0 ∘C .
What is a normal substance with a low boiling point?
The normal boiling point is high for liquids with strong intermolecular attractions and low for liquids with weak intermolecular attractions. Helium has the lowest normal boiling point, 4.2 kelvin (−268.9°C). What increases boiling point?
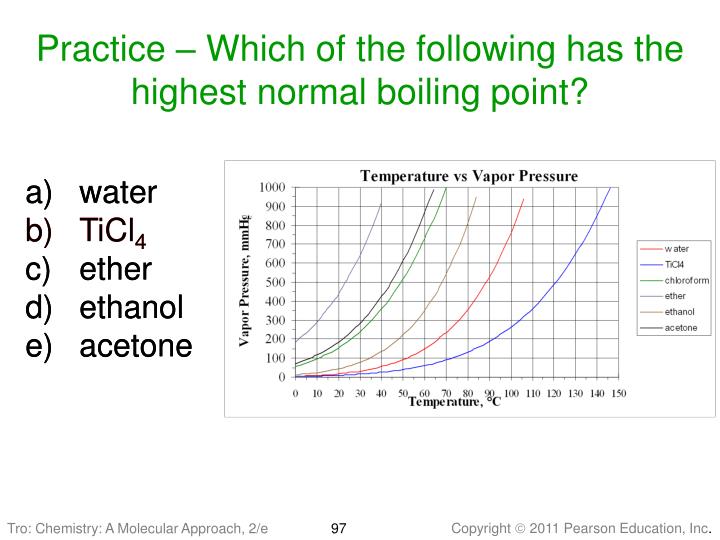
How do you find the normal boiling point of a substance?
To find the normal boiling point of a liquid, a horizontal line is drawn from the left at a pressure equal to standard pressure. At whatever temperature that line intersects the vapor pressure curve of a liquid is the boiling point of that liquid.
What is the normal boiling point of a?
The normal boiling point of a liquid is the temperature at which its vapor pressure is equal to one atmosphere (760 torr).
What is the normal melting point of the substance?
The normal melting point of a substance is its melting point at a pressure of 1 atm. For a pure substance, the freezing point of the liquid equals the melting point of the solid. For pure water, the normal melting point is 0.0024 °C.
What is the boiling point of any substance?
The boiling point of a pure substance is the temperature at which the substance transitions from a liquid to the gaseous phase. At this point, the vapor pressure of the liquid is equal to the applied pressure on the liquid. The boiling point at a pressure of 1 atmosphere is called the normal boiling point.
How do you find the normal boiling point on a phase diagram?
0:421:27Boiling Point from PVT Diagram (Example) - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo I draw a horizontal line at one atmosphere. And then if I draw a vertical. Line. ThisMoreSo I draw a horizontal line at one atmosphere. And then if I draw a vertical. Line. This intersection should correspond to the normal boiling point and so halfway in between these.
What is meant by normal boiling point and normal melting point?
Now the normal melting and the normal boiling points, by definition are the temperatures at which the solid changes to liquid, and the liquid changes to gas at ONE ATMOSPHERE . So simply draw a horizontal line on the y axis, and note the x intercepts of the transitions to give these properties.
How do you find the normal melting point and normal boiling point on a phase diagram?
The normal melting and boiling points are those when the pressure is 1 atmosphere. These can be found from the phase diagram by drawing a line across at 1 atmosphere pressure.
What is the normal boiling point of co2?
-109.2°F (-78.46°C)Carbon dioxide / Boiling point
What is the normal boiling point of ccl4?
170.1°F (76.72°C)Carbon tetrachloride / Boiling point
What is the boiling point of a substance in Celsius?
100 °CThe Celsius temperature scale was defined until 1954 by two points: 0 °C being defined by the water freezing point and 100 °C being defined by the water boiling point at standard atmospheric pressure.
What is the normal boiling point of ethanol?
173.1°F (78.37°C)Ethanol / Boiling point
Normal vs Regular Boiling Point
Normal boiling point is defined as the boiling point at sea level or 1 atmosphere. Kroeger & Gross / Getty Images
Normal Boiling Point Definition
Normal boiling point is the temperature at which a liquid boils at 1 atmosphere of pressure. It's different from the simple definition of boiling point in that the pressure is defined. The normal boiling point is a more useful value when comparing different liquids, since boiling is affected by altitude and pressure.
What is the boiling point of a substance?
The boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal to the surrounding atmospheric pressure, thus facilitating transition of the material between gaseous and liquid phases . All boiling points below are normal/atmospheric boiling points: they give the temperature at which the vapor pressure ...
What is the boiling point of a molal solution of salt?
For a 1.0 molal solution of salt (containing 58.44 grams of salt per kg of water), the boiling point is raised by 1.0 degrees Celsius.