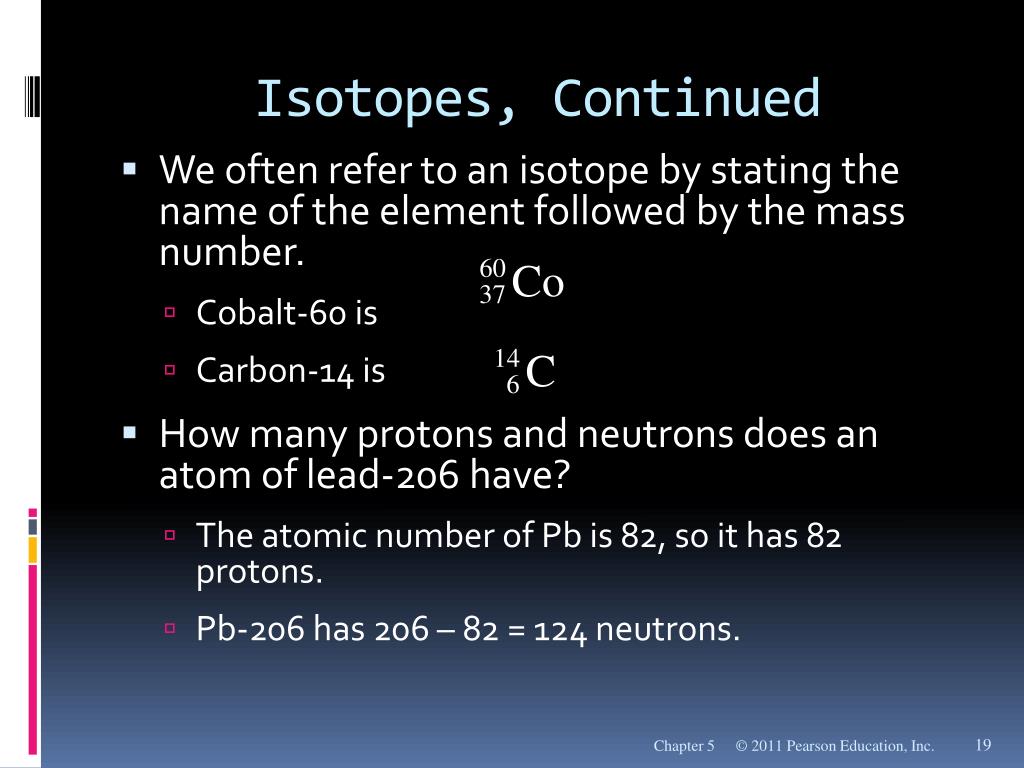
How many protons, neutrons and electrons does mercury have?
| Element Name | Mercury |
| Symbol | Hg |
| Atomic number | 80 |
| Atomic weight (average) | 200.59u |
| Protons | 80 |
How many neutrons are in one atom of mercury?
One atom of Mercury has 121 neutrons. To find the number of neutrons in Mercury, you need to first find its atomic number on the Periodic Table of Click to see full answer. Similarly, it is asked, what is the number of protons in mercury? One may also ask, how many neutrons are in Mercury 202? 122 neutrons
What is the formula to get the number of neutrons?
N = M – n N = number of N eutrons M = atomic M ass n = atomic n umber
- N = number of N eutrons
- M = atomic M ass
- n = atomic n umber
What does the number of neutrons determine?
The number of neutrons in atoms don't really determine anything, they are simply there to balance out the attracting charges between the protons and electrons, to prevent the atom from collapsing. The most that the number of neutrons in atoms can determine are the different isotopes of an element.
How many nuetrons are in the nucleus of mercury?
Moreover, how many neutrons does Mercury 202 have? 122 neutrons . What element has a mass number of 200? Gold, isotope of mass 200 | Au - PubChem. ... Take note that the nucleus of an atom is composed of protons and neutrons. And the number of particles present in the nucleus is referred as mass number (Also, called as atomic mass). ...
See more

What is the number of electrons of mercury?
2,8,18,32,18,2Mercury / Electrons per shell
What is the electrons and neutrons in mercury?
NameMercuryAtomic Mass200.59 atomic mass unitsNumber of Protons80Number of Neutrons121Number of Electrons809 more rows
How many neutrons are in mercury 80?
Hg20480 has 80 protons 80 electrons and 124 neutrons.
How many number of protons does mercury have?
80Mercury / Atomic number
How do u find neutrons?
To find the number of neutrons, subtract the number of protons from the mass number. number of neutrons=40−19=21.
How do you find number of electrons?
The number of electrons in a neutral atom is equal to the number of protons. The mass number of the atom (M) is equal to the sum of the number of protons and neutrons in the nucleus. The number of neutrons is equal to the difference between the mass number of the atom (M) and the atomic number (Z).
Does mercury have 121 neutrons?
Since you know from its atomic number that Mercury has 80 protons, to find the number of neutrons you'll subtract 80 (the number of protons) from 201 (the total number of neutrons and protons). There are 121 neutrons in one atom of Mercury.
What has 27 protons and 33 neutrons?
CobaltAnswer and Explanation: Based on the number of protons, we see that the nuclide in question is Cobalt. Cobalt has 27 protons and can exist as one of its isotopes with 33 neutrons.
What has 56 protons and 80 neutrons?
NameBariumAtomic Number56Atomic Mass137.327 atomic mass unitsNumber of Protons56Number of Neutrons819 more rows
What has 37 protons and 52 neutrons?
NameRubidiumDensity1.532 grams per cubic centimeterNormal PhaseSolidFamilyAlkali MetalsPeriod59 more rows
What has 52 protons and 78 neutrons?
Tellurium is a chemical element with symbol Te and atomic number 52.
How many atoms are in mercury?
So, 1 atom is present in each mercury molecule.
How many neutrons are in an atom of Hg 201?
The atomic number is the number of protons in an atom's nucleus, so we can tell right away that an atom of mercury contains 80 protons. The mass number (201) is the total number of protons and neutrons. So, there must be 201 - 80 = 121 neutrons.
How many atoms are in mercury?
So, 1 atom is present in each mercury molecule.
What is the charge in mercury?
Mercury's atomic number is 80. In nature, mercury has 3 possible conditions of electrical charge, or valence states. Elemental mercury (Hg0) has no electric charge. Mercury is also found in two positively charged, or cationic, states, Hg2+ (mercuric) and Hg1+(mercurous).
Does mercury have more protons or electrons?
Mercury has more protons and electrons than tin. the number of protons and electrons in an atom is dependent on the atomic number of that element.
How do neutrons stabilize the nucleus?
Neutrons stabilize the nucleus, because they attract each other and protons , which helps offset the electrical repulsion between protons. As a result, as the number of protons increases, an increasing ratio of neutrons to protons is needed to form a stable nucleus. If there are too many or too few neutrons for a given number of protons, the resulting nucleus is not stable and it undergoes radioactive decay. Unstable isotopes decay through various radioactive decay pathways, most commonly alpha decay, beta decay, or electron capture. Many other rare types of decay, such as spontaneous fission or neutron emission are known. It should be noted that all of these decay pathways may be accompanied by the subsequent emission of gamma radiation. Pure alpha or beta decays are very rare.
How many electrons are in a neutral atom of mercury?
Therefore, the number of electrons in neutral atom of Mercury is 80. Each electron is influenced by the electric fields produced by the positive nuclear charge and the other (Z – 1) negative electrons in the atom.
What is the Pauli exclusion principle?
It is the Pauli exclusion principle that requires the electrons in an atom to occupy different energy levels instead of them all condensing in the ground state. The ordering of the electrons in the ground state of multielectron atoms, starts with the lowest energy state (ground state) and moves progressively from there up the energy scale until each of the atom’s electrons has been assigned a unique set of quantum numbers. This fact has key implications for the building up of the periodic table of elements.
What is the periodic table?
The periodic table is a tabular display of the chemical elements organized on the basis of their atomic numbers , electron configurations, and chemical properties. The electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. Knowledge of the electron configuration of different atoms is useful in understanding the structure of the periodic table of elements.
How many protons does mercury have?
Mercury is a chemical element with atomic number 80which means there are 80 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10-19 coulombs.
What are the two forces that make up the nucleus?
Atomic nuclei consist of protons and neutrons, which attract each other through the nuclear force, while protons repel each other via the electric force due to their positive charge. These two forces compete, leading to various stability of nuclei. There are only certain combinations of neutrons and protons, which forms stable nuclei.
What is the number of neutrons in an atom?
The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol N. Neutron number plus atomic number equals atomic mass number: N+Z=A. The difference between the neutron number and the atomic number is known as the neutron excess: D = N – Z = A – 2Z.
Why is Cinnabar used?
Cinnabar would yield up its mercury simply on heating in a crucible, and the metal fascinated people because it was a liquid that would dissolve gold. The ancients used in on a large scale to extract alluvial gold from the sediment of rivers. The mercury dissolved the gold which could be reclaimed by distilling off the mercury.
How much mercury is in food?
Every mouthful of food we eat contains a little mercury. Our daily intake is less than 0.01 milligrams (about 0.3 grams in a lifetime), and this we can cope with easily. However, in much higher doses it is toxic and one form of mercury – methylmercury – is particularly dangerous.
Where did the mercury come from?
The Almadén deposit in Spain provided Europe with its mercury. In the Americas, it was the Spanish conquerors who exploited the large deposits of cinnabar at Huancavelica in order to extract gold. In 1848 the miners of the Californian Gold Rush used mercury from the New Almaden Mines of California.
What is density in science?
Density is the mass of a substance that would fill 1 cm 3 at room temperature. Relative atomic mass. The mass of an atom relative to that of carbon-12. This is approximately the sum of the number of protons and neutrons in the nucleus.
What is the symbol of mercury?
Image explanation. The image is of a traditional alchemical symbol for mercury. This is also an astrological symbol for the planet Mercury. The dragon or serpent in the background comes from early alchemical drawings and is often associated with the element.
How are elements organized into blocks?
Elements are organised into blocks by the orbital type in which the outer electrons are found. These blocks are named for the characteristic spectra they produce: sharp (s), principal (p), diffuse (d), and fundamental (f). The number of protons in an atom.
What is the vertical column in the periodic table?
A vertical column in the periodic table. Members of a group typically have similar properties and electron configurations in their outer shell. A horizontal row in the periodic table. The atomic number of each element increases by one, reading from left to right.
How to find the number of protons in mercury?
To find the number of protons in mercury mercury, first locate the element on the periodic table. Next, find the atomic number which is located above the element 's symbol. Since mercury mercury 's atomic number is 80 80, Hg Hg has 80 80 protons.
What is the mass number of an atom?
The mass number is the number of particles in an atom 's nucleus. The nucleus is made up of protons and neutrons.
What is 80+N in math?
Rewrite the equation as 80+N = 201 80 + N = 201.