What is the octet rule, and how is it used in covalent bonding? – Octet rule states that atoms lose, gain, or share electrons to achieve a stable configuration of 8 valence electrons (octet). It is used in covalent bonding when the atom share electrons to achieve octet.
Does the octet rule apply to covalent bonding?
Since only the valence electrons are shown in these diagrams, the attainment of a noble-gas structure is easily recognized as the attainment of a full complement of eight electron dots (an octet) around each symbol. In other words covalent as well as ionic compounds obey the octet rule.
What is the octet rule and how is it used in covalent bonding?
What is the octet rule, and how is it used in covalent bonding? - Octet rule states that atoms lose, gain, or share electrons to achieve a stable configuration of 8 valence electrons (octet). It is used in covalent bonding when the atom share electrons to achieve octet.
What is octet rule explain?
What is the Octet Rule? The octet rule dictates that atoms are most stable when their valence shells are filled with eight electrons.
How does ionic and covalent bonding relate to the octet rule?
Ionic Bonding Just as with the covalent compounds, each ion wishes to form an octet and be like the nearest noble gas. Sometimes it is easier for the element to gain electron(s) (anions) to produce the octet and sometimes it is easier for the element to lose electron(s) (cations).
How do you find the octet rule?
There are two ways in which atoms can satisfy the octet rule. One way is by sharing their valence electrons with other atoms. The second way is by transferring valence electrons from one atom to another.
How do you write the octet rule?
0:262:36Lewis Structures: Octet Rule (Example) - YouTubeYouTubeStart of suggested clipEnd of suggested clipThe octet rule for the bromine tascam has two electrons co2 we know carbon has four oxygen has sixMoreThe octet rule for the bromine tascam has two electrons co2 we know carbon has four oxygen has six so if I draw the six valence electrons for oxygen.
Why is there an octet rule?
Atoms follow the octet rule because they always seek the most stable electron configuration. Following the octet rule results in completely filled s- and p- orbitals in an atom's outermost energy level. Low atomic weight elements (the first 20 elements) are most likely to adhere to the octet rule.
What is the octet rule in ionic bonding?
In the formation of a chemical bond, atoms lose, gain or share valence electrons to complete their outer shell and attain a noble gas configuration. • This tendency of atoms to have eight electrons in their outer shell is known as the octet rule.
How does the octet rule apply to ionic and covalent bonds quizlet?
Atoms with covalent bonds satisfy the octet rule by sharing electrons. Nonmetals with ionic bonds satisfy the octet rule by gaining electrons.
What is octet rule quizlet?
Octet Rule. States that atoms must attain 8 electrons in outermost energy level to become stable. Subatomic Particles. Particles SMALLER than an atom.
How does the octet rule apply to covalent bonds How is this different from how it applies to ionic bonds?
If atoms have similar electronegativities (the same affinity for electrons), covalent bonds are most likely to occur. Because both atoms have the same affinity for electrons and neither has a tendency to donate them, they share electrons in order to achieve octet configuration and become more stable.
How do you use octet rule?
0:104:45The Octet Rule: Help, Definition, and Exceptions - YouTubeYouTubeStart of suggested clipEnd of suggested clipWhen elements have eight electrons in their outer shell they have an octet.MoreWhen elements have eight electrons in their outer shell they have an octet.
What is the Octet Rule?
The octet rule dictates that atoms are most stable when their valence shells are filled with eight electrons. It is based on the observation that the atoms of the main group elements have a tendency to participate in chemical bonding in such a way that each atom of the resulting molecule has eight electrons in the valence shell. The octet rule is only applicable to the main group elements.
Which elements obey the octet rule?
In general, the elements that obey this rule include the s-block elements and the p-block elements (except hydrogen, helium, and lithium). The octet rule can be observed in the bonding between the carbon and oxygen atoms in a carbon dioxide molecule, ...
How many valence electrons does oxygen have?
Yes, each oxygen atom in the O 2 molecule is surrounded by a total of 8 valence electrons. Oxygen has a total of 6 electrons in the valence shell. In order to obtain a stable octet, the two oxygen atoms share a total of four electrons via a double bond.
How many valence electrons does phosphorus hold?
If all the phosphorus-chlorine bonds in a PCl 5 molecule are covalent, it would imply that the phosphorus molecule is violating the octet rule by holding a total of 10 valence electrons. The formation of five bonds by the phosphorus molecules can be explained by the sp 3 d hybridization in PCl 5.
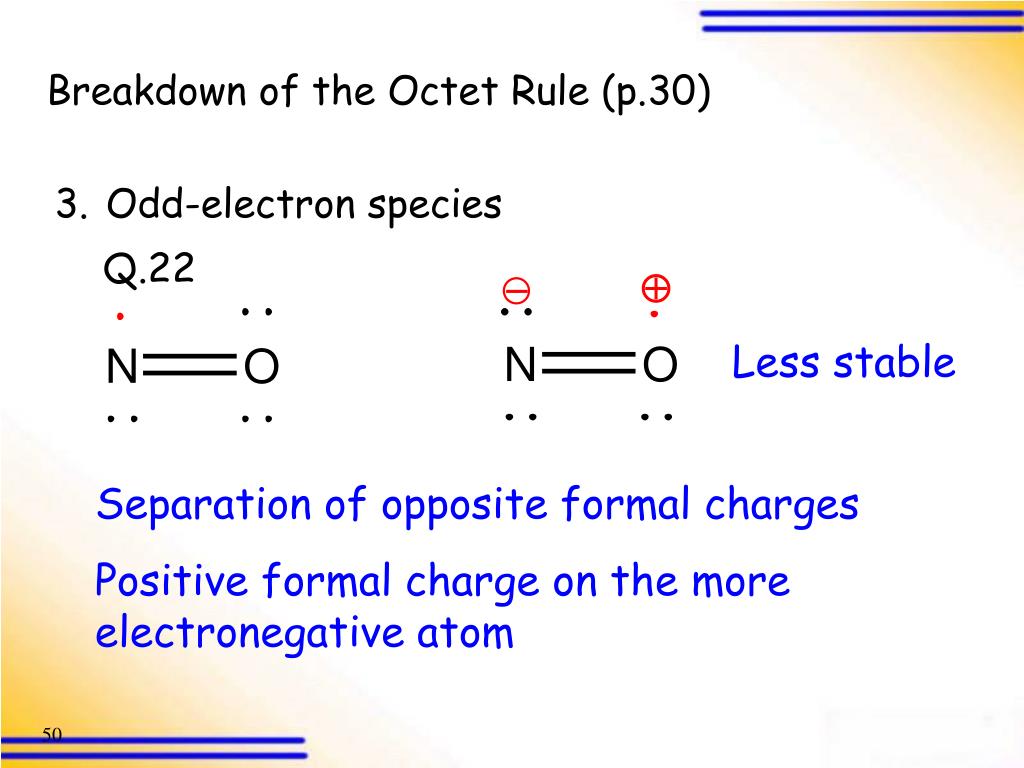
What is an ion, atom, or a molecule containing an unpaired valence electron?
An ion, atom, or a molecule containing an unpaired valence electron is called a free radical. These species disobey the octet rule. However, they are very unstable and tend to spontaneously dimerize.
How many electrons does a transition atom hold?
Due to the presence of a d-orbital, the transition elements do not obey the octet rule. The valence shells of these atoms can hold 18 electrons.
What is the bond between NaCl and NaCl?
NaCl (Sodium Chloride) This compound features an ionic bond between the sodium ion (Na +) and the electronegative chloride ion (Cl – ). The chlorine atom holds 7 electrons in its valence shell and can attain an octet configuration by gaining an electron. The outermost shell of sodium has one electron. If it loses this electron, the second shell ...
Why is the octet rule important in covalent bonding?
The octet rule is important in covalent bonding because sharing electrons gives both atoms a full valence shell.
How many electrons does an oxygen atom have?
For example, an oxygen atom has six electrons in its valence shell. The most the shell can hold is eight. Two oxygen atoms can share their valence electrons as shown below.
What happens when atoms can't reach their full outer shell?
If atoms can’t achieve a full outer shell by transferring electrons, they resort to sharing. In this way, each atom can count the shared electrons as part of its own valence shell. This sharing of electrons is covalent bonding.
What does the octet rule mean?
The octet rule states that elements gain or lose electrons to attain an electron configuration of the nearest noble gas. Here is an explanation of how this works and why elements follow the octet rule.
Why do elements follow the octet rule?
Why Elements Follow the Octet Rule. Atoms follow the octet rule because they always seek the most stable electron configuration. Following the octet rule results in completely filled s- and p- orbitals in an atom's outermost energy level. Low atomic weight elements (the first 20 elements) are most likely to adhere to the octet rule.
How many electrons does chlorine have?
Chlorine, for example, has seven electrons in its outer electron shell. Chlorine readily bonds with other elements so that it can have a filled energy level, like argon; +328.8 kJ per mole of chlorine atoms are released when chlorine acquires a single electron.
Which element has a complete outer electron shell?
Noble gases have complete outer electron shells, which make them very stable. Other elements also seek stability, which governs their reactivity and bonding behavior. Halogens are one electron away from filled energy levels, so they are very reactive.
Why do we use Lewis electron dot diagrams?
Lewis electron dot diagrams may be drawn to help account for the electrons participating in a chemical bond between elements. A Lewis diagram counts the valence electrons. Electrons shared in a covalent bond are counted twice. For the octet rule, there should be eight electrons accounted for around each atom.
What Is The Octet Rule?
Examples
- A few examples detailing the chemical bonding of atoms in compliance with the octet rule can be found in this subsection.
Exceptions to The Octet Rule
- Not all elements and compounds follow the octet rule. Some of the exceptions to this rule are listed below. 1. An ion, atom, or a molecule containing an unpaired valence electron is called a free radical. These species disobey the octet rule. However, they are very unstable and tend to spontaneously dimerize. 2. Since the first shell can only accommodate two electrons, elements …
Frequently Asked Questions
- How is the Octet Rule Useful?
The chemical behaviour of the main group elements can be predicted with the help of the octet rule. This is because the rule only involves ‘s’ and ‘p’ electrons, where the octet corresponds to an electron configuration ending with s2p6. These elements tend to form bonds in order to obtain s… - Do the Oxygen Atoms in O2 Molecules Have Octet Configurations?
Yes, each oxygen atom in the O2molecule is surrounded by a total of 8 valence electrons. Oxygen has a total of 6 electrons in the valence shell. In order to obtain a stable octet, the two oxygen atoms share a total of four electrons via a double bond.