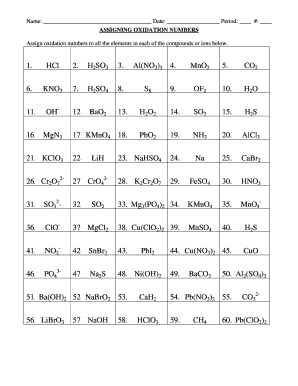
How to find oxidation number?
Take phosphoric acid (H 3 PO 4 ) as an example :
- First the Lewis formula is recorded.
- Then the electrons are assigned to the atoms according to electronegativity
- The oxidation number can then be calculated based on the valence electrons. Example: Oxygen normally has 6 valence electrons (VI. Main group). ...
How to assign oxidation numbers?
Assigning oxidation numbers to organic compounds The oxidation state of any chemically bonded carbon may be assigned by adding -1 for each bond to more electropositive atom (H, Na, Ca, B) and +1 for each bond to more electronegative atom (O, Cl, N, P), and 0 for each carbon atom bonded directly to the carbon of interest.
How to calculate oxidation number practice problems?
- Oxidation number can be positive or zero or negative
- Oxidation number has to be an integer as the number of electrons can only be an integer.
- Oxidation number cannot be fractional
- The oxidation number is the same as the oxidation state.
What are the oxidation number rules?
The rules for oxidation numbers are: The total of all the atoms in a molecule’s oxidation numbers equals zero. An atom’s oxidation number in its most basic form is always zero. In their compounds, the oxidation number of alkali metals (Li, Na ,K ,Rb ,Cs) is always +1. In their compounds, the oxidation number of alkaline earth metals (Be ,Mg ,Ca ,Sr ,Ba) is always +2.
What is the oxidation number of no G?
0:000:50How to find the Oxidation Number for N in NO (Nitrogen monoxide)YouTubeStart of suggested clipEnd of suggested clipWe know that oxygen is usually minus two and if oxygen is minus two in order for this to add up toMoreWe know that oxygen is usually minus two and if oxygen is minus two in order for this to add up to zero the nitrogen has to be a plus two. And that's the oxidation number for nitrogen.
What is the principal oxidation number of gallium?
+3Chemical properties Gallium is found primarily in the +3 oxidation state.
How do you get the oxidation number?
1:2931:15How To Calculate Oxidation Numbers - Basic Introduction - YouTubeYouTubeStart of suggested clipEnd of suggested clipState of each oxygen atom in this ion. You could write an equation as two oxygen atoms with a totalMoreState of each oxygen atom in this ion. You could write an equation as two oxygen atoms with a total charge of negative two. So individually each oxygen atom has a charge of minus one.
What is the oxidation state of gallium in gallium cl2?
Gallium (Ga) can have an oxidation state of +1, +2, and +3. And, oxidation state of chlorine is -1. So, according to cross multiplication method when 2 chlorine atoms are attached to Ga then oxidation state of Ga is +2.
What ion does Ga form?
Gallium cationPubChem CID105145Molecular FormulaGa+3SynonymsGallium cation gallium(3+) Gallium (III) ion Gallium, ion(3+) UNII-F7K5MP217W More...Molecular Weight69.723DatesModify 2022-06-05 Create 2005-08-012 more rows
What is the number of electron of gallium?
31 electronsGallium atoms have 31 electrons and the shell structure is 2.8. 18.3.
What is the oxidation number of o2?
-2Oxygen usually has an oxidation number of -2. Exceptions include molecules and polyatomic ions that contain O-O bonds, such as O2, O3, H2O2, and the O22- ion. 8. The elements in Group VIIA often form compounds (such as AlF3, HCl, and ZnBr2) in which the nonmetal has a -1 oxidation number.
What is the oxidation number of all elements?
NOTE: * is for rare oxidation numberAtomic NumberElementOxidation numbers6Carbon-4 , -3 , -2 , -1 , 0 , +1 , +2 , +3 , +47Nitrogen-5 , -4 , -3 , -2 , -1 , 0 , +1 , +2 , +38Oxygen-2 , -1 , 0 , +1 , +29Fluorine-1 , 016 more rows
What is the oxidation number for h2o?
It is important to note that oxidation number always refers to each individual atom in the compound, not to the total for that element. For example, in H2O, the total positive "charge" for both hydrogen atoms will be +2 (which balances with the -2 from oxygen), but each hydrogen has an oxidation number of +1.
How do you find the oxidation number of an ion?
0:061:34How to Find Oxidation Numbers for an Ion - YouTubeYouTubeStart of suggested clipEnd of suggested clipNumber the chlorine in the center that's normally minus one except when it's bonded to things likeMoreNumber the chlorine in the center that's normally minus one except when it's bonded to things like well chlorine bromine iodine and then oxygen.
How many oxidation numbers are there in the periodic table?
Some elements in the periodic table have only one oxidation number or two oxidation numbers. But some have lot of oxidation numbers. Oxidation number of element in a compound can be positive or negative or may be zero. In sodium compounds, sodium only forms +1 oxidation number. But some types of atoms such as chlorine form various oxidation numbers ...
What is the oxidation number of sodium?
In sodium compounds, sodium only forms +1 oxidation number . But some types of atoms such as chlorine form various oxidation numbers like -1, 0, +1, +3, +5, +7 oxidation numbers in compounds. In this tutorial, we discuss about some important facts of oxidation states and oxidation numbers in periodoc table including s block, p block and d block.
What are the oxidation numbers of P block elements?
Some p block elements have lot of oxidation numbers such as chlorine (-1, 0, +1, +3, +5, +7) and sulfur (-2, 0, +4, +6) . And some have limited oxidation numbers like fluorine (-1).
How many electrons does sulfur have?
Sulfur gives its all last six electrons to make sulfuric acid molecule (+6 oxidation state). Chlorine can give seven electrons to make chloric acid to show +7 oxidation number. Chlorine can take one electron to form chloride anion.(-1 oxidation state). Sulfur can take two electrons to form sulfide anion.
When an element has not combined or do not form a compound, what is the oxidation number?
When an element has not combined or do not form a compound. When an element has not combined, it's oxidation number is 0. Ex: oxidation number of Au is 0. When an element has combined with same kind element. When an element has combined with same kind element, it's oxidation number becomes 0 .
Can oxidation numbers be positive or negative?
In this chapter, we discuss very important facts about oxidation numbers with examples with different compounds. Oxidation number can be positive or negative. We know metalsrelease electrons to form positive ions. Therefore metals always form positiveoxidation numbers.
Is oxygen a positive or negative number?
Oxygen has the second highest electronegative value in periodic table. So in most occasions, oxidation number of oxygen is negative. Oxygen only forms positive oxidation numbers when it combine with fluorine.
What is the oxidation number of hydrogen?
Hydrogen has an oxidation number of +1 when combined with non-metals, but it has an oxidation number of -1 when combined with metals. The algebraic sum of the oxidation numbers of elements in a compound is zero. The algebraic sum of the oxidation states in an ion is equal to the charge on the ion.
What is the oxidation number of alkaline earth metals?
The alkaline earth metals (group II) are always assigned an oxidation number of +2. Oxygen almost always has an oxidation number of -2, except in peroxides (H 2 O 2) where it is -1 and in compounds with fluorine (OF 2) where it is +2.
What is the oxidation state of an atom?
The oxidation state of an atom is the charge of this atom after ionic approximation of its heteronuclear bonds. The oxidation number is synonymous with the oxidation state. Determining oxidation numbers from the Lewis structure (Figure 1a) is even easier than deducing it from the molecular formula (Figure 1b).
What is the oxidation number of a monatomic ion?
The oxidation number of a monatomic ion equals the charge of the ion. Fluorine in compounds is always assigned an oxidation number of -1. The alkali metals (group I) always have an oxidation number of +1. The alkaline earth metals (group II) are always assigned an oxidation number of +2.
What is the abbreviation for a group in which a carbon atom is attached to the rest of
Bonds between atoms of the same element (homonuclear bonds) are always divided equally. Figure 1. Different ways of displaying oxidation numbers of ethanol and acetic acid. R is an abbreviation for any group in which a carbon atom is attached to the rest of the molecule by a C-C bond.
What is the oxidation number of oxygen?
Oxygen almost always has an oxidation number of -2, except in: peroxides (e.g. H2O2) where it is -1. compounds with fluorine (e.g. OF2) where it is +2. The oxidation number of H is +1 when combined with more electronegative elements (e.g. non-metals) it is -1 in when combined with less electronegative elements (e.g. metals).
What is the oxidation number of a group 1 element?
The oxidation number of a Group 1 element in a compound is +1. The alkali metals (group I) always have an oxidation number of +1. The oxidation number of a Group 2 element in a compound is +2.
What is the sum of oxidation numbers in a neutral compound?
The sum of oxidation numbers in a neutral compound is 0. The sum of the oxidation numbers in a monatomic ion is equal to the overall charge of that ion. The oxidation number of fluorine is always –1.
What is the oxidation number?
In simple words, the oxidation number is the number assigned to the components in a chemical combination. The oxidation number is the total number of electrons that atoms in a molecule can share, lose, or acquire while establishing chemical interactions with atoms of another element. In this article, let’s learn everything about oxidation numbers in detail.
What is the oxidation number of oxygen in most of its oxides?
Oxidation Number Rule 7. The oxidation number of oxygen in most of its oxides is − 2 except in peroxide, superoxides, oxyfluoride, and ozonide’s. Example: In Na2O, the oxidation number of O is − 2. In Mg0, the oxidation number of O is − 2.
Why is the oxidation number used interchangeably with the oxidation state?
It is because the stock notation of oxidation numbers is the basis of the periodic property, electronegativity. An atom in a molecule can assign to negative, positive or zero oxidation number by considering its environment.
What is the sum of oxidation numbers of all the atoms in a molecule?
The sum of oxidation numbers of all the atoms in a molecule is equal to zero. Example: In KMnO4, oxidation number of K is + 1, the oxidation number of Mn is + 7 and oxidation number of oxygen is − 2.
What is the maximum oxidation number of an element?
The maximum oxidation number of any element is equal to its group number except in the case of oxygen and fluorine. Example: The oxidation number of sulphur in H2S2O8, K2S2O8, S2O – 28 and H2SO5 is + 6 due to the presence of a peroxide bond.
What is the oxidation number of carbonyls?
The oxidation number of metals in all Metal carbonyls is zero. Example: In Ni(CO)4, the oxidation number of Ni is zero. Note: In NCl3, nitrogen oxidation number is − 3, and Cl is + 1 because nitrogen is smaller in size when compared to chlorine.
What is the average oxidation state number of sulphur in Na2S2O3?
So the average Oxidation state number of sulphur in Na2S2O3, is + 2. In CaOCl2 the oxidation number of one chlorine is − 1 and another chlorine + 1. So the average oxidation number of chlorine is zero. Structure of CaOCl2 and Na2S2O3.