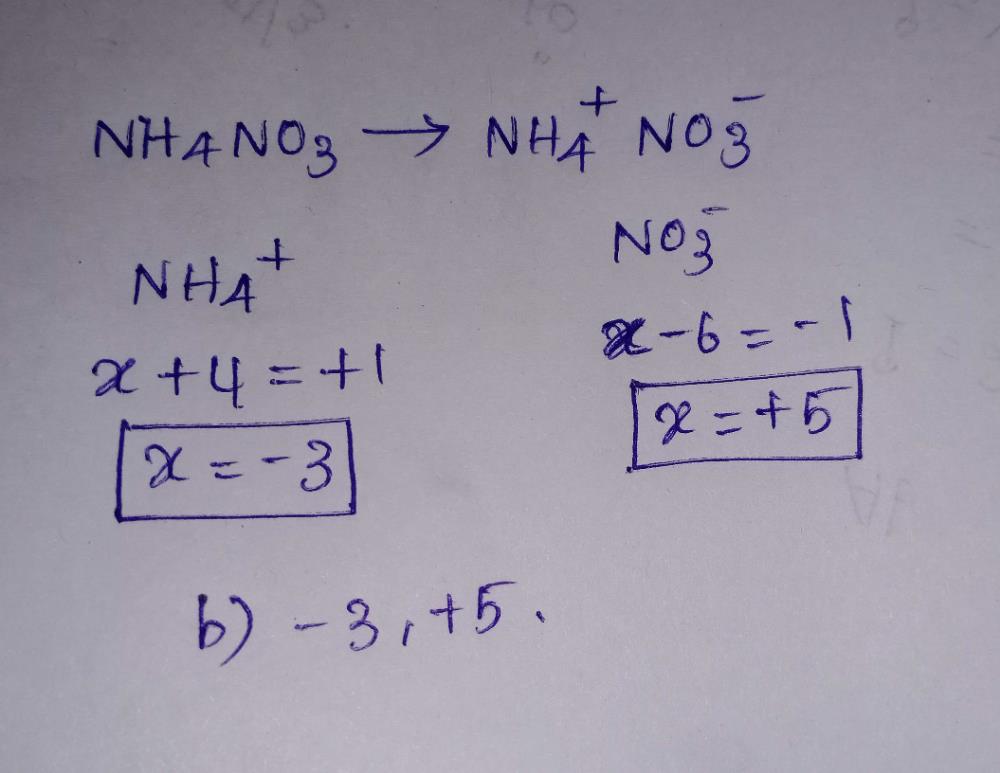
How to calculate oxidation numbers?
Using Lewis diagrams
- Draw the Lewis diagram for the compound, including all valence electrons.
- Assign the electrons from each bond to the more negative bond partner identified by ionic approximation. Homonuclear bonds should be divided equally.
- The resulting atom charges then represent the oxidation state for each atom.
How to find oxidation number?
Take phosphoric acid (H 3 PO 4 ) as an example :
- First the Lewis formula is recorded.
- Then the electrons are assigned to the atoms according to electronegativity
- The oxidation number can then be calculated based on the valence electrons. Example: Oxygen normally has 6 valence electrons (VI. Main group). ...
How do you calculate oxidation?
S has an oxidation number of 6 in Na 2 SO 4.
- (Sum of known oxidation numbers) + (unknown oxidation number you are solving for) = (charge of the compound)
- -6 + S = 0
- S = 0 + 6
- S = 6. S has an oxidation number of 6 in Na 2 SO 4.
What are the rules for oxidation numbers?
What are the rules for determining oxidation numbers and examples?
- The oxidation number of a free element is zero.
- The oxidation number of a monatomic ion equals the charge on the ion.
- The sum of oxidation numbers in a neutral compound is zero. ...
- The oxidation number of the Group IA element is +1 and Group IIA element is +2,
See more
What is the charge of NH4Cl?
Ammonium chloride (NH4+ Cl-) is a molecule. It's overall electrical charge is zero, and its atoms are held together by both covalent bonds and an ionic bond. The ammonium cation is not a molecule by itself because its net electrical charge is +1 (it is a cation).
What is the oxidation number for hydrogen in NH4Cl?
0:501:39How to find the Oxidation Number for N in NH4Cl (Ammonium chloride)YouTubeStart of suggested clipEnd of suggested clipIs minus three and these are the oxidation numbers for nh4cl ammonium chloride you should end upMoreIs minus three and these are the oxidation numbers for nh4cl ammonium chloride you should end up with zero.
How do you find the oxidation number?
Calculating Oxidation NumbersAny free element has an oxidation number equal to zero.For monoatomic ions, the oxidation number always has the same value as the net charge corresponding to the ion.The hydrogen atom (H) exhibits an oxidation state of +1. ... Oxygen has an oxidation of -2 in most of its compounds.More items...•
What is the oxidation number of NH4 2S?
Oxidation State of (NH4)2S - Ammonium Sulfide The oxidation number of N in (NH4)2S is -3.
What is the oxidation state of ammonium?
-3The oxidation state of nitrogen in ammonia molecule is -3.
What is the valency of nitrogen in nh4cl?
But the fourth hydrogen atom is coordinately bound to nitrogen atom. Therefore, the fourth bond in ammonium ion is a coordinate covalent bond and this is also a maximum covalency of nitrogen. Thus, according to the definition of covalency the total number of covalency in ammonium ion is four.
What is oxidation number with example?
The oxidation number of ions which comprise of only one atom is equal to the actual charge on the ion. In most of the compounds, the oxidation number of oxygen is –2. There are two exceptions here. Oxygen is bonded to fluorine- Example, dioxygen difluoride where the oxygen atom is allocated an oxidation number of +1.
What is the oxidation number for h2o?
0:001:31How to find the Oxidation Numbers for H2O (Water) - YouTubeYouTubeStart of suggested clipEnd of suggested clipYou can consider the oxidation number for water h2o to be zero since it's a neutral compound. And byMoreYou can consider the oxidation number for water h2o to be zero since it's a neutral compound. And by that we mean if we add up the oxidation numbers for each of the elements in water we'd get zero.
What is the oxidation number of co2?
+4As a result, carbon in CO2 has an oxidation number of +4.
What is the oxidation number of N in NH4 +?
- 3Hence, the oxidation number of N in NH 4 + ion = - 3.
How do you find the oxidation number of nitrogen in NH4+?
0:151:26How to find the Oxidation Number for N in the NH4+ ion. (Ammonium ion)YouTubeStart of suggested clipEnd of suggested clip4 plus a minus 3 essentially 4 minus 3 that equals plus 1.. So this minus three that's the oxidationMore4 plus a minus 3 essentially 4 minus 3 that equals plus 1.. So this minus three that's the oxidation. Number on the nitrogen.
What is the cation in NH4 2S?
cation ammoniumAnswer and Explanation: The compound (NH4)2S ( N H 4 ) 2 S is formed from the cation ammonium (NH+4 N H 4 + ) and the sulfide anion (S2− ). Compounds made from cations and anions are ionic compounds.
What is the oxidation state of nh4 2cro4?
The oxidation number of Cr in (NH4)2CrO4 is +6. The oxidation number of H in (NH4)2CrO4 is +1....Oxidation State of (NH4)2CrO4 - Ammonium Chromate.ElementOOxidation Number (Avg)-2Atoms-2 (×4)Count4Electronegativity3.6103 more columns
What is the oxidation number of nh4 2so4?
x+4×1=+1; ⇒x=−3.
What is the oxidation number of h2 o2?
In hydrogen peroxide (H2O2), the oxidation state or oxidation number of oxygen is −1.
Which compound contains nitrogen with an oxidation number of +3 nh4cl?
Nitrogen trichloride is a chemical compound. It contains nitrogen in its -3 oxidation state. It contains nitrogen and chloride ions.
How to calculate oxidation of NH4NO3?
By taking NH4 as a whole in (NH4)NO3, then oxidation no. of NH4NO3 can be calculated as = oxidation no. Of NH4 + oxidation no. Of N + oxidation no. Of O3
What is the oxidation state of chlorine?
So the oxidation state of chlorine in this molecule is -1. The oxidation state of hydrogen is almost always +1. Now what’s left is only the oxidation state of chlorine. We can find it by doing a simple equation where the sum of the charges is 0.
What is the process of forming NO2?
This is called Berkland and Eyde process. During rain when there is lightening the atmostpheric nitrogen and oxygen combines forming NO (nitric oxide). Which again reacts with oxygen forming nitrogen dioxide NO2. This is washed from atmosphere to soil because of rain forming nitrates in soil which helps in soil fertility. The nitrogen is oxidized during the process.
Which ion forms an ionic bond with chloride?
Further this ammonium ion due to a positive charge forms an ionic bond with chloride ion (Cl–).
Is SCl2 a covalent compound?
SCl2 is a covalent compound, in which sulfur makes a single bond to each of two chlorine atoms. Thus, each atom in the structure “has an octet.” But chlorine’s electronegativity (3.2) is higher than that of sulfur (2.8). so the oxidation state model for “bookkeeping electrons” assigns the shared electrons to the chlorines. Each chlorine has an oxidation state of -1 and the sulfur has an oxidation state of +2.
Is nitrogen a covalent bond?
There is actually a coordinate bond (which is actually a kind of covalent bond in which only one of the participating atoms donate both of their electrons to form a covalent bond) between lone pair of nitrogen in ammonia (NH3) and a proton (H+ ion),which hence creates an ammonium ion (NH4+).
Is NH4 negatively charged?
Furthermore, Cl ions are negatively charged meaning they usually come with a -1 charge. Couple the -1 charge with the +! charge of NH4 and the overall charge of the molecule is zero. Therefore N in this molecule has a charge of -3 (the lone pair it shares doesn’t add on to the charge )