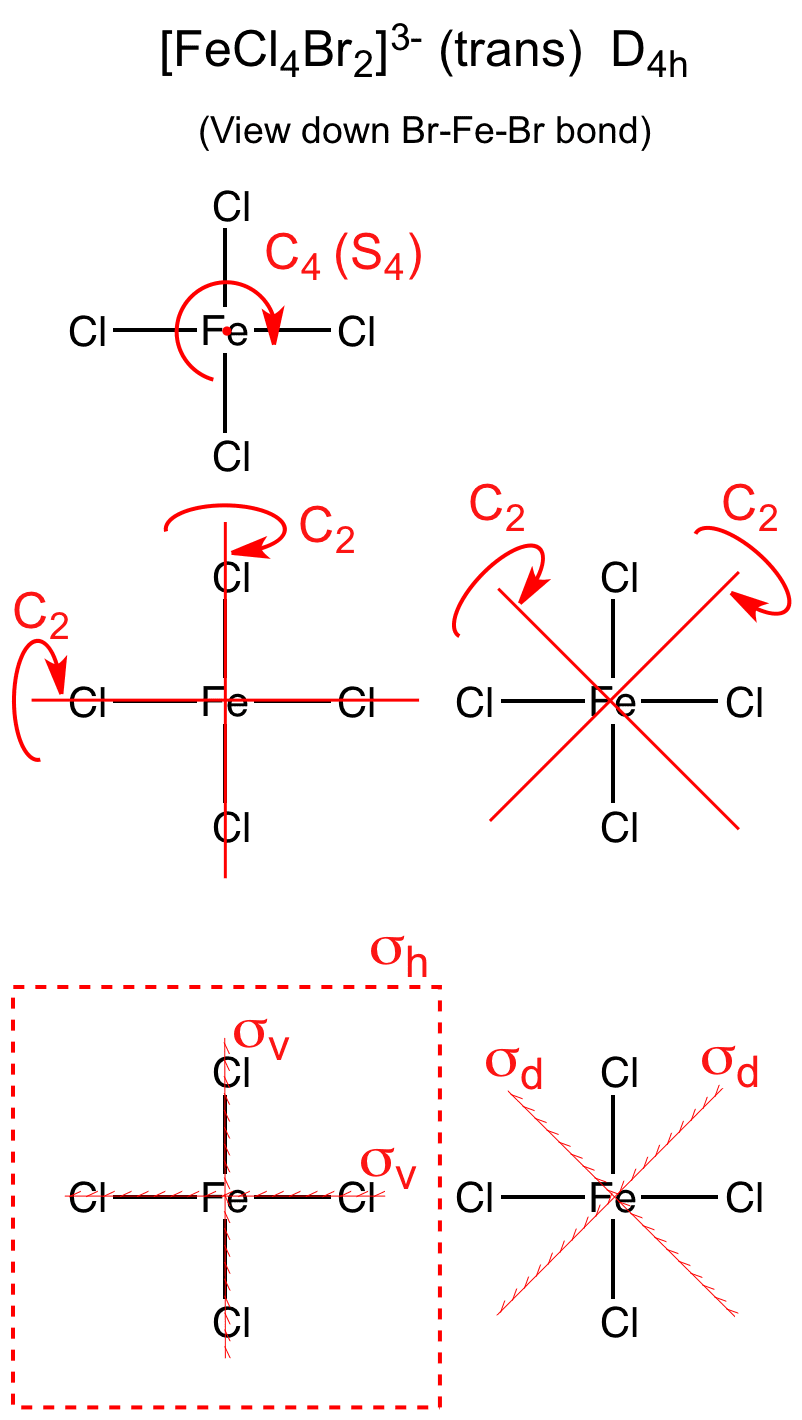
What is the point group of POCl3 C3v?
Jun 25, 2020 · What is the point group of pocl3? Phosphorous Oxychloride C C3v point groups contain one C3 rotation and 3σv planes. Click to see full answer. Correspondingly, what is the point group of PCl3? PCl3 is trigonal pyramidal (use VSEPR theory) and so possesses the same symmetry elements as NH3 in worked example 3.2. These are E, C3 and 3 v.
What is the bond length of POCl3?
At low concentrations the free acids resulting from the hydrolysis of phosphoryl trichloride will be neutralised quickly by body fluids. The resulting phosphate and chloride ions are natural components of food and ubiquitously found in living tissues and are not expected to pose a hazard. At high concentrations, which exceed the buffer capacity of body fluids the acids will …
What is the point group of PtCl4 2?
Phosphorous Oxychloride C 3v. Phosphorous Oxychloride C. 3v. 1 model in this collection. Use getProperty "modelInfo" or getProperty "auxiliaryInfo" to inspect them. C 3v point groups contain one C 3 rotation and 3σ v planes.
What is the name of XeF4 POCl3?
Oct 31, 2011 · Determine the point group of each molecule. Identify any molecules that are chiral. POCL 3 , BFClI, PFClI, SF 5 Cl, CO, XeF 2, Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 1,2,3,4- tetrachlorocyclobutane: it is a non linear molecule ...
Is pocl3 contain C3 axis?
Phosphoryl chloride (commonly called phosphorus oxychloride) is a colourless liquid with the formula POCl 3. It hydrolyses in moist air releasing phosphoric acid and fumes of hydrogen chloride. It is manufactured industrially on a large scale from phosphorus trichloride and oxygen or phosphorus pentoxide.
What is the the shape molecular geometry of pocl3?
1:202:03POCl3 Molecular Geometry / Shape and Bond Angles - YouTubeYouTubeStart of suggested clipEnd of suggested clipWe know that pocl3 is tetrahedral and the bond angles are around that 109.5.MoreWe know that pocl3 is tetrahedral and the bond angles are around that 109.5.
What is the point group of SF4?
SF4 is disphenoidal in shape and has a main C2 rotation axis, with two σv mirror planes. Hence it's point group is C2v.Mar 2, 2021
How do you find the point group?
1:365:52Assigning Point Groups to Molecules - YouTubeYouTubeStart of suggested clipEnd of suggested clipIf an inversion Center is not present then the molecule will be assigned the TD point group as isMoreIf an inversion Center is not present then the molecule will be assigned the TD point group as is the case for methane. If an inversion Center is present.
Is POCl3 a polar molecule?
The molecular geometry of POCl3 is tetrahedral with asymmetric charge distribution around the central atom. Therefore this molecule is polar.
Why is P central atom in POCl3?
Step 1: Connect the atoms with single bonds. The less electronegative is the phosphorous atom. Hence, the P atom is going to be the central atom. Recall that electronegativity decreases as we move away from the fluorine atom in the periodic chart.Jul 8, 2013
What is the point group of h2o2?
Hydrogen Peroxide C. 2 Hydrogen Peroxide only contains a C2 axis. Hence it belongs to the C2 point group.
What is the point group of CH3Cl?
C3v point groupExplanation: CH3Cl belongs to the C3v point group. It has the symmetry element E, a C3 axis, and three σv planes.Jun 12, 2018
What is the point group of ph3?
PH3 is a trigonal pyramidal molecule with C3v molecular symmetry. The length of the P−H bond is 1.42 Å, the H−P−H bond angles are 93.5°.
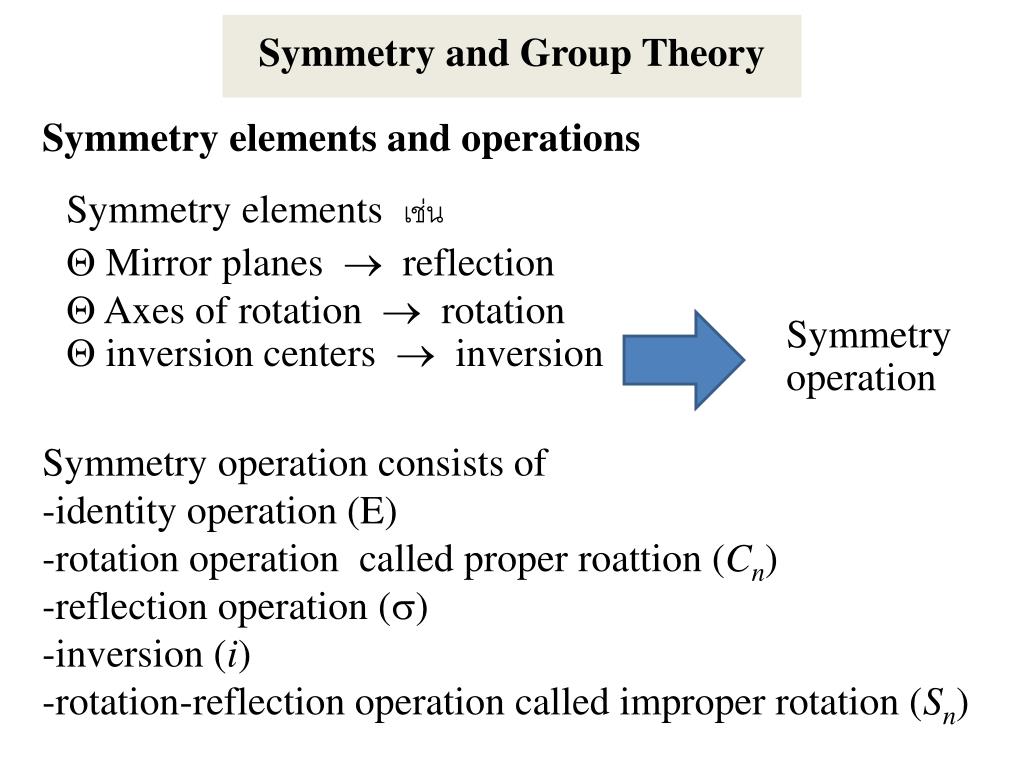
What is a point group in chemistry?
A Point Group describes all the symmetry operations that can be performed on a molecule that result in a conformation indistinguishable from the original. Point groups are used in Group Theory, the mathematical analysis of groups, to determine properties such as a molecule's molecular orbitals.Oct 14, 2020
What is point group and space group?
Point groups and space groups are terms described under crystallography. The crystallographic point group is a set of symmetry operations all of which leave at least one point unmoved. A space group is the 3D symmetry group of a configuration in space.Mar 15, 2018
What is a point group in physics?
point group, also called Crystal Class, in crystallography, listing of the ways in which the orientation of a crystal can be changed without seeming to change the positions of its atoms.
What is the formula for phosphoryl chloride?
Phosphoryl chloride (commonly called phosphorus oxychloride) is a colourless liquid with the formula P O Cl 3. It hydrolyses in moist air releasing phosphoric acid and fumes of hydrogen chloride. It is manufactured industrially on a large scale from phosphorus trichloride and oxygen or phosphorus pentoxide. It is mainly used to make phosphate esters such as tricresyl phosphate .
What is POCl 3?
POCl 3 can also act as a Lewis base, forming adducts with a variety of Lewis acids such as titanium tetrachloride : The aluminium chloride adduct (POCl 3 ·AlCl 3) is quite stable, and so POCl 3 can be used to remove AlCl 3 from reaction mixtures, for example at the end of a Friedel-Crafts reaction .
What is phosphoryl chloride used for?
In one commercial application, phosphoryl chloride is used in the manufacture of phosphate esters. Triarylphosphates such as triphenyl phosphate and tricresyl phosphate are used as flame retardants and plasticisers for PVC. Trialkylphosphates such as tributyl phosphate are used as liquid–liquid extraction solvents in nuclear reprocessing ...
What is the boiling point of POCl 3?
With a freezing point of 1 °C and boiling point of 106 °C, the liquid range of POCl 3 is rather similar to water. Also like water, POCl 3 autoionizes, owing to the reversible formation of POCl 2+ ,Cl − .
What is tributyl phosphate used for?
Trialkylphosphates such as tributyl phosphate are used as liquid–liquid extraction solvents in nuclear reprocessing and elsewhere. In the semiconductor industry, POCl 3 is used as a safe liquid phosphorus source in diffusion processes. The phosphorus acts as a dopant used to create n-type layers on a silicon wafer.
What is the reaction of POCl 3?
In the Vilsmeier-Haack reaction, POCl 3 reacts with amides to produce a "Vilsmeier reagent", a chloro- iminium salt, which subsequently reacts with electron-rich aromatic compounds to produce aromatic aldehydes upon aqueous work-up.
What is a point group?
In geometry, a point group is a group of geometric symmetries ( isometries) that keep at least one point fixed. Point groups can exist in a Euclidean space with any dimension, and every point group in dimension d is a subgroup of the orthogonal group O ( d ). Point groups can be realized as sets of orthogonal matrices M ...
Do point groups have infinite families?
Discrete point groups in more than one dimension come in infinite families, but from the crystallographic restriction theorem and one of Bieberbach's theorems, each number of dimensions has only a finite number of point groups that are symmetric over some lattice or grid with that number.
What is the Coxeter notation?
Coxeter notation offers a bracketed notation equivalent to the Coxeter diagram, with markup symbols for rotational and other subsymmetry point groups. Reflection groups are necessarily achiral (except for the trivial group containing only the identity element).

Overview
Physical properties
With a freezing point of 1 °C and boiling point of 106 °C, the liquid range of POCl3 is rather similar to water. Also like water, POCl3 autoionizes, owing to the reversible formation of POCl2 ,Cl .
Structure
Like phosphate, phosphoryl chloride is tetrahedral in shape. It features three P−Cl bonds and one strong P=O double bond, with an estimated bond dissociation energy of 533.5 kJ/mol. On the basis of bond length and electronegativity, the Schomaker-Stevenson rule suggests that the double bond form is dominant, in contrast with the case of POF3. The P=O bond involves th…
Chemical properties
POCl3 reacts with water to give hydrogen chloride and phosphoric acid:
O=PCl3 + 3 H2O → O=P(OH)3 + 3 HCl
Intermediates in the conversion have been isolated, including pyrophosphoryl chloride, P2O3Cl4.
Upon treatment with excess alcohols and phenols, POCl3 gives phosphate esters:
O=PCl3 + 3 ROH → O=P(OR)3 + 3 HCl
Preparation
Phosphoryl chloride can be prepared by many methods. Phosphoryl chloride was first reported in 1847 by the French chemist Adolphe Wurtz by reacting phosphorus pentachloride with water.
The commercial method involves oxidation of phosphorus trichloride with oxygen:
2 PCl3 + O2 → 2 POCl3
An alternative method involves the oxidation of phosphorus trichloride with potassium chlorate:
Uses
In one commercial application, phosphoryl chloride is used in the manufacture of phosphate esters. Triarylphosphates such as triphenyl phosphate and tricresyl phosphate are used as flame retardants and plasticisers for PVC. Trialkylphosphates such as tributyl phosphate are used as liquid–liquid extraction solvents in nuclear reprocessing and elsewhere.
Further reading
• Handbook of Chemistry and Physics (71st ed.). Ann Arbor, MI: CRC Press. 1990.
• Stecher, Paul G. (1960). The Merck Index of Chemicals and Drugs (7th ed.). Rahway: Merck & Co. OCLC 3653550.
• Wade, L. G., Jr (2005). Organic Chemistry (6th ed.). Upper Saddle River, NJ: Pearson/Prentice Hall. p. 477.