Key Points
- Functional groups are often used to “functionalize” a compound, affording it different physical and chemical properties than it would have in its original form.
- Functional groups will undergo the same type of reactions regardless of the compound of which they are a part; however, the presence of certain functional groups within close proximity can limit reactivity.
What are six examples of functional groups?
What are some examples of functional groups?
- Hydroxyl Functional Group.
- Aldehyde Functional Group.
- Ketone Functional Group.
- Amine Functional Group.
- Amino Functional Group.
- Amide Functional Group.
- Ether Functional Group.
- Ester Functional Group.
What do all functional groups have in common?
functional groups is used to describe the pieces or parts of a drug molecule. The key point here is that each individual group within a drug molecule can serve to provide one or more specifi c roles, tasks, or functions. As evidenced by functional groups A and B, the same functional group—a carboxylic acid in this case—can serve different roles
What are functional groups and their properties?
Functional Groups and Their Properties. What is a functional group? • A structural arrangement of atoms which determines the physical and chemical properties in a given molecule • Organic molecules are grouped into organic families based on the functional groups present
What are functional groups responsible for?
Functional Groups, in the field of organic chemistry, are the substituent atoms or groups of atoms that are attached to specific molecules. These moieties (the part of the molecule which can be found in many other molecules as well) are responsible for the chemical reactions that the molecule they are attached to participate in.
What is the primary function of functional groups?
Functional Groups in Organic Compounds Functional groups are important in chemistry because they are the portion of a molecule that is capable of characteristic reactions. They, therefore, determine the properties and chemistry of many organic compounds.
What is the significance of functional groups quizlet?
The functional group gives the molecule its properties, regardless of what molecule contains it; they are centers of chemical reactivity. The functional groups within a molecule need to be identified when naming.
What are functional groups in biology quizlet?
The seven functional groups that are most important in the chemistry of life: hydroxyl, carbonyl, carboxyl, amino, sulfhydryl, phosphate, methyl groups.
What describes a functional group biology?
A functional group is a specific group of atoms within a molecule that is responsible for a characteristic of that molecule. Many biologically active molecules contain one or more functional groups.
What is a functional group group of answer choices?
Functional groups are groups of atoms that occur within organic molecules and confer specific chemical properties to those molecules.
How do functional groups affect biological function?
Each type of organic molecule has its own specific type of functional group. Functional groups in biological molecules play an important role in the formation of molecules like DNA, proteins, carbohydrates, and lipids.
How do you identify functional groups?
1:366:0910.1 Identifying functional groups (SL) - YouTubeYouTubeStart of suggested clipEnd of suggested clipThis molecule contains an alkyne a functional group if we look at the condensed structural formulaMoreThis molecule contains an alkyne a functional group if we look at the condensed structural formula we can see that we have one carbon atom that is not bonded to any hydrogen atoms.
How many functional groups are there in organic chemistry?
What are the four functional groups? In biological molecules, some of the essential functional groups include hydroxyl, methyl, carbonyl, carboxyl, amino, phosphate, and sulfhydryl groups. These groups play a significant role in forming molecules such as DNA, proteins, carbohydrates, and lipids.
What are Functional Groups?from byjus.com
Functional Groups are a “particular grouping of components in which the distinctive chemical reactions of these molecules are accountable”.
What are functional groups in organic chemistry?from byjus.com
A functional group in organic chemistry is a collection of atoms within molecules which bind together to react in predictable ways. Examples of functional groups include the group hydroxyl, ketone, amine, and ether.
What happens when a highly electronegative functional group is attached to a less electronegative atom or molecule?from byjus.com
In the scenario wherein a highly electronegative functional group is attached to a less electronegative atom or molecule, a polarity arises which enables the initially nonpolar molecule to be soluble in water or other aqueous environments.
What functional groups contain carbon-oxygen bonds?from byjus.com
Many uncommon groups with complex compositions such as acetal groups (RCH (OR’) (OR’’), or ketal groups RC (OR’) (OR”)R”. Nitrogen-Containing Functional Groups The substituent groups that contain nitrogen may also contain carbon-oxygen bonds.
What is the first carbon atom attached to the functional group?from byjus.com
The first carbon atom attached to the functional group is called alpha carbon; the second, beta carbon; the third, gamma carbon, and so forth. Similarly, a functional group can be called principal, secondary, or tertiary, depending on whether it is connected to one, two , or three atoms of carbon.
What happens when the functional groups of a solute and a solvent interact well?from byjus.com
If the functional groups of the solute and the solvent interact well, the solubility increases. For example, since sugar and water both contain the -OH (hydroxyl) group, sugar can be easily dissolved in water. In the scenario wherein a highly electronegative functional group is attached to a less electronegative atom or molecule, ...
How can organic chemists tell a lot about a molecule?from thoughtco.com
Organic chemists can tell a lot about a molecule by the functional groups that make up a molecule. Any serious student should memorize as many as they can. This short list contains many of the most common organic functional groups. It should be noted that the R in each structure is a wildcard notation for the rest of the molecule's atoms.
What is a Functional Group?
A group of atoms forming a component of a molecule that accounts for a particular function or chemical behavior is called a functional group. These moieties or sections can be found in different molecules. In fact, a moiety can identify a compound and introduce it into a particular genre or classification of similar compounds.
List of Functional Groups
Now that we have defined what a functional group in chemistry is, let us find out the types with proper examples.
What are Functional Groups?
Functional Groups are a “particular grouping of components in which the distinctive chemical reactions of these molecules are accountable”.
How does functional group affect solubility?
If the functional groups of the solute and the solvent interact well, the solubility increases. For example, since sugar and water both contain the -OH (hydroxyl) group, sugar can be easily dissolved in water.
What happens when a highly electronegative functional group is attached to a less electronegative atom or molecule?
In the scenario wherein a highly electronegative functional group is attached to a less electronegative atom or molecule, a polarity arises which enables the initially nonpolar molecule to be soluble in water or other aqueous environments.
What is the functional group that is bound to the central atom called?
In a coordination complex, the functional group that is bound to the central atom is said to be a ligand .
Which functional group has a carbonyl carbon atom?
For example, the amide functional group has the formula R- (CO)-NR 2 and therefore has a carbonyl carbon which is bonded to a nitrogen atom, which is in turn bonded to two other alkyl groups. Some common functional groups that contain nitrogen are tabulated below along with the suffixes for their nomenclature.
How is chemical synthesis designed?
The process of chemical synthesis, in which chemical reactions are intentionally executed in order to obtain a specific compound, can be designed by understanding the properties of various functional groups.
Which functional group has an ionic charge?
The hydrocarbon functional groups may have an ionic charge on them. The positively charged structures are referred to as carbocations whereas the negatively charged hydrocarbons are called carbanions.
Role of Functional Groups
Functional groups distinguish a molecule by determining its reactivity and how it interacts with other molecules. A functional group gives an organic substance a unique property that it would not have otherwise. A molecule’s functional groups determine the following:
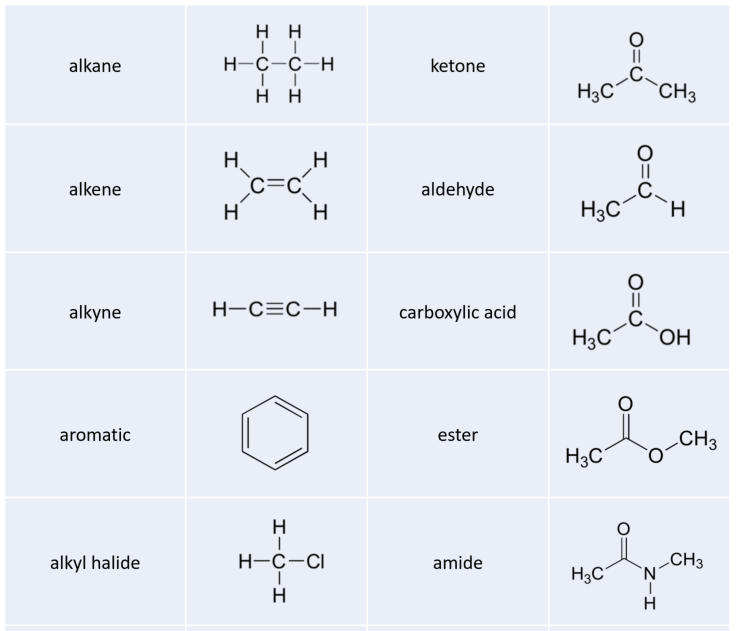
Alkenes and Alkynes
Alkenes are unsaturated hydrocarbons with one or more double bonds between adjacent carbon atoms. The general molecular formula for the homologous series of alkenes is CnH2n, where n is the number of carbon atoms in one molecule. The general structure of an alkene is as follows:
Benzene
Benzene, having the molecular formula C 6 H 6, is a type of organic chemical compound. The benzene molecule is made up of three C=C double bonds that alternate with single bonds in a planar ring, each with one hydrogen atom linked to it.
Alcohol
Alcohol is a type of chemical substance with one or more hydroxyl groups. A hydroxyl group is made up of -OH. A carbon is single-bonded to a -OH group in the alcohol functional group. The general molecular formula for the homologous series of alkynes is CnH2n+1OH, where n is the number of carbon atoms in one molecule.
Ether
Ethers are a class of functional group chemical compounds that contain an ether group, which is an oxygen atom linked to two alkyl or aryl groups. They are represented by the generic formula R-O-R′, where R and R′ indicate alkyl or aryl groups.
Amine
Amine is an organic derivative of ammonia (NH 3 ). When the hydrogen atom (s) in NH 3 are replaced by R groups, amine is formed. Nitrogen atoms with single bonds to hydrogen and carbon describe amines.
Aldehydes
Aldehydes are carbonyl compounds with a hydrogen and an alkyl (or aromatic) group attached to them. Aldehyde can only exist at the end of a structure since H must be attached on one side of the C=O group. The generic formula RCHO is used to express them.
Why are functional groups important?
Just because the main reason is that they form the fundamental of a organic molecule, Functional groups are important.
What makes a molecule special?
The functional groups make a molecule special deciding on its reactivity and how it interacts with other molecules.
Why does the functional group approach work?
The functional group approach " works" because the properties and reaction chemistry of a particular functional group (FG) can be remarkably independent of environment.
What is functional group approach in organic chemistry?
The functional group approach " works" because the properties and reaction chemistry of a particular functional group (FG) ...
What are primary amines?
Primary amines can be shown in text as: RNH2 Primary amines are basic functions that can be protonated to the corresponding ammonium ion. Primary amines are also nucleophilic. Secondary amine. Secondary amines have a pair of alkyl or aromatic groups, and a hydrogen, attached to a nitrogen atom.
How many alkyl groups are in tertiary amines?
Tertiary amines have three alkyl or aromatic groups attached to a nitrogen atom. Tertiary amines can be shown in text as: R3N Tertiary amines are basic functions that can be protonated to the corresponding ammonium ion. Tertiary amines are also nucleophilic. Nitrile.
Which group is a super function?
Carbonyl function. The carbonyl group is a super function because many common functional groups are based on a carbonyl, including: aldehydes, ketones, carboxylic acids, esters, amides, acyl (acid) chlorides, acid anhydrides. Ester. Esters have a pair of alkyl or aromatic groups attached to a carbonyl + linking oxygen function.
Which drug has multiple functional groups and chiral centers?
A multifunctional entity like the drug molecule morphine may have several functional groups and chiral centers:
Is 2-chlorohexane a single function?
2-chlorohexane. The rule is that functions assume their distinct identity when separated by –CH 2 – groups. Thus, the carbonyl, C=O, and hydroxy, OH, of a carboxylic acid, RCOOH, are part of a single function and are NOT "alcohol-plus-ketone":
How do functional groups influence solubility?
The size of the molecule and functional groups present on the molecule also determine whether or not it will be soluble in a particular solvent. Many of the same functional groups that raise the boiling point of a compound (carbonyl and hydroxyl groups) also increase its solubility in water.
What is the significance of functional groups quizlet?
The functional group gives the molecule its properties, regardless of what molecule contains it; they are centers of chemical reactivity. The functional groups within a molecule need to be identified when naming.
Why are functional groups important to biology?
Functional groups are collections of atoms that attach the carbon skeleton of an organic molecule and confer specific properties. Functional groups in biological molecules play an important role in the formation of molecules like DNA, proteins, carbohydrates, and lipids.
What is the effect of functional group?
In organic chemistry, a functional group is a specific group of atoms or bonds within a compound that is responsible for the characteristic chemical reactions of that compound. The same functional group will behave in a similar fashion, by undergoing similar reactions, regardless of the compound of which it is a part.
What is the functional group of water?
The chemical composition of water is H2O it does not contain any functional groups. Of Course it has an OH group, but this is not identified as an alcohol group, as we see it in different types of alcohols (methanol.
Why do functional groups increase water solubility?
Any functional group that can donate a hydrogen bond to water (eg. alcohols, amines) will significantly contribute to water solubility. Any functional group that can only accept a hydrogen bond from water (eg. ketones, aldehydes, ethers) will have a somewhat smaller but still significant effect on water solubility.
What is a functional group group of answer choices?
Functional groups are groups of atoms that confer specific properties to hydrocarbon (or substituted hydrocarbon) chains or rings that define their overall chemical characteristics and function.
What is functional group?
Functional groups are specific groups of atoms within organic molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction(s) regardless of the size of the molecule it is a part of.
Which chain is usually attached to a functional group?
The functional groups are usually attached to a hydrocarbon chain which is non-polar.
How does oxygen help a molecule?
In many functional groups, oxygen helps establish the polarity of the molecule. In some groups, oxygen acts as a reducing agent by losing electrons from compounds while in other groups, it acts as an oxidizing agent by gaining electrons to form a bond.
Model of Human Occupations (MOHO)
The Model of Human Occupations (MOHO) is a model that describes how humans generate and modify their occupations in interaction with environment, which presents a dynamic open cycle system of human actions.
Allen's Cognitive Disabilities Model (CDM)
The Allen’s Cognitive Disabilities Model (CDM) emphases on the integration of the cognitive functional ability and the level of activities that clients are able and willing to perform. Interventions using this model can take place individually or in group.
Occupational Performance Process Model
The Occupational Performance Process Model is based on the concepts of occupation and client-centered practice; that is, therapists should solve the clients’ occupational performance problems through the client-centered approach.
Developmental Frame of Reference
The developmental frame of reference (FOR) suggests that development is sequential, and behaviors are primarily influenced by the extent to which an individual has mastered and integrated the previous stages.
Model of Seven-Level Hierarchy of Family-Therapist Involvement
This model presents a hierarchy of family-therapist involvement in occupational therapy services, with associated attitudes, specific knowledge, and skills that enable therapists to operate at each level.

What Is A Functional Group?
- A group of atoms forming a component of a molecule that accounts for a particular function or chemical behavior is called a functional group. These moieties or sections can be found in different molecules. In fact, a moiety can identify a compound and introduce it into a particular genre or classification of similar compounds. When we define a func...
Classification of Functional Groups
- Functional groups can be classified in the following ways. 1. Hydrocarbons This type of functional group contains only hydrogen and carbon as constituent elements. They are commonly represented with ‘R’. They are also called hydrocarbyl groups. The bonding between the carbon atoms can be single, double, or triple. The carbon and hydrogen bonds are always single. These …
List of Functional Groups
- Now that we have defined what a functional group in chemistry is, let us find out the types with proper examples. 1. Alcohol (-OH) The suffix of this functional group is -ol and the organic compound formed is called an alcohol. Example - Methyl alcohol or methanol. 1. Aldehyde (-CHO) In this functional group, a hydrogen and an oxygen atom are bonded with a carbon atom leaving …
Table of Contents
What Are Functional Groups?
- Two molecules having different sizes but the same functional groups will take part in chemical reactions that are similar or exactly the same. The presence of a functional group in a molecule implies that the behaviour and the chemical reactions of the molecule in question can be predicted in a systematic fashion. The process of chemical synthesis, in which chemical reactio…
Role of Functional Groups
- Organic Chemistry Functional Groups
The manner in which the functional groups indulge in a chemical reaction can be further modified with the help of other functional groups, and these groups can also be interconverted. A few functional groups involving carbon are illustrated below. Therefore, it can be understood that fun…
Nomenclature of Common Functional Groups
- The common functional groups, along with the prefix and the suffix which must be used in their nomenclature is provided in this subsection. Additionally, a brief description of the constitution of each of these groups is also provided.