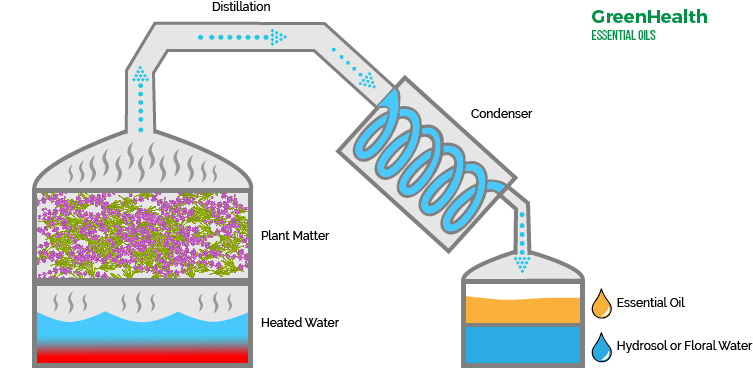
The principle behind the steam distillation process is that when the heating of a mixture of two or more immiscible liquids takes place, the vapour pressure exerted by the system increases. This is because it now becomes the sum of the vapour pressures of all of the components of the mixture combined together.
What are the most efficient methods of distillation?
Types of distillation in detail
- Simple distillation. Here a liquid mixture is heated in a conical flask. ...
- Fractional distillation. This is similar to simple distillation with the only addition of a fractionating column. ...
- Steam distillation. ...
- Vacuum distillation. ...
- Azeotropic distillation. ...
- Double distillation. ...
- Triple distillation. ...
Who refined the process of steam distillation?
The process of steam distillation was refined by the. Ottoman Empire in the late 14th century and early 15th century. Avicenna described the process of distillation in his treatise, “On Canon of Medicine.” He recommended using either glass or earthenware still due to their apparent ability to produce higher-quality distilled water and ...
How to make essential oil using steam distillation?
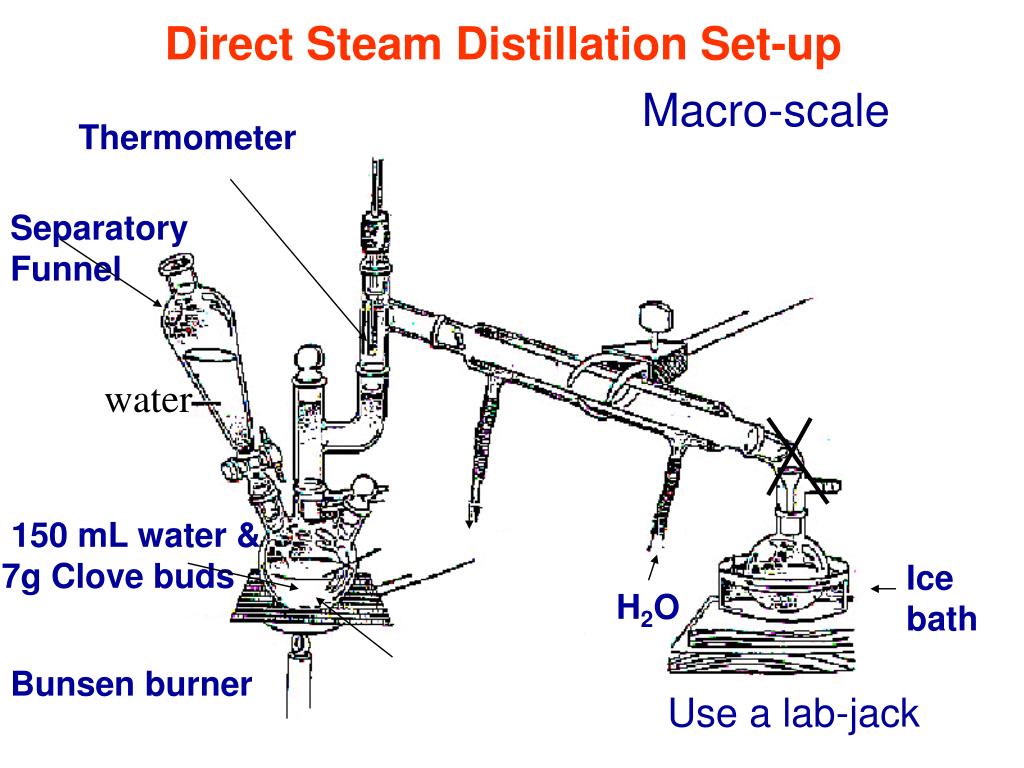
How to Distill Essential Oils: the 7 basic steps to steam distillation:
- Steam is created from boiling water
- Passing through plant biomass, the steam breaks up the plant micro particles.
- Separating the volatile from non-volatile organic compounds, both rise with the steam.
- A condenser cools the steam, which is transformed back to water.
- In a separator, the volatile principles (i.e. ...
What are steps involve the process of distillation?
The process of distillation begins with heating a liquid to boiling point. The liquid evaporates, forming a vapor. The vapor is then cooled, usually by passing it through pipes or tubes at a lower ...
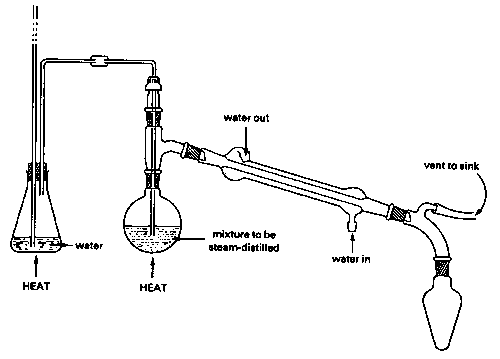
What is the basic principle of distillation experiment?
The use of distillation to separate the components in a mixture is based on the principle that the boiling liquid in the vial and the vapor produced have a different composition.
What is distillation and its principle?
Distillation is a simple physical separation method based on the difference in boiling temperatures of the components of a solution. The solution is heated at a selective boiling temperature that forces one of the components to enter the gaseous phase and is thus separated from the solution.
What law is steam distillation based on?
Dalton's law of partial pressureSo, the whole process is based on the formula of Dalton's law of partial pressure.
What are the 3 steps of distillation?
The distillation process generally involves three main steps: The conversion of the desired liquid from a mixture into vapour. The condensation of the purified liquid. The collection of the condensed liquid.
What is the purpose of distillation?
Distillation is the process of vaporizing and condensing a liquid to purify or concentrate a substance or to separate a volatile substance from less volatile substances. It is the oldest method of water purification.
How is steam distillation different from simple distillation?
Steam distillation is analogous to simple distillation, the main difference being that steam (or water) is used in the distilling flask along with the material to be distilled.
Why is water used in steam distillation?
Steam or water is added to the distillation apparatus, lowering the boiling points of the compounds. The goal is to heat and separate the components at temperatures below their decomposition point.
What mixtures are separated through steam distillation?
Steam distillation is a separation technique that harnesses the low boiling point property of immiscible mixtures. It is predominately used to separate temperature-sensitive organic molecules from a non-volatile contaminant. The organic molecule must be immiscible in water.
What is called distillation?
distillation, process involving the conversion of a liquid into vapour that is subsequently condensed back to liquid form. It is exemplified at its simplest when steam from a kettle becomes deposited as drops of distilled water on a cold surface.
What is a distillation in chemistry?
Distillation is a powerful technique for separating the component substance from a miscible fluid mixture by means of selective evaporation and condensation.
What is distillation and its application?
Distillation is the process of separating components of a mixture based on different boiling points. Examples of uses of distillation include purification of alcohol, desalination, crude oil refining, and making liquefied gases from air. Humans have been using distillation since at least 3000 BC in the Indus valley.
What is distillation and types of distillation?
Distillation, or classical distillation, is the process of separating the components or substances from a liquid mixture by using selective boiling and condensation. Dry distillation is the heating of solid materials to produce gaseous products (which may condense into liquids or solids).
What is steam distillation used for?
The procedure is referred to as steam distillation if water is used as one of the immiscible liquids. It is often used to purify liquids that at th...
How is steam distillation different from simple distillation?
This behaviour happens because boiling requires a lower vapour pressure, which can be accomplished at a lower temperature. Steam distillation is si...
Which compounds are steam volatile?
Distillation that distinguishes the target product from other materials or impurities also purifies volatile organic compounds. Under steam distill...
What is steam distillation of essential oils?
The most common way to remove aromatic compounds (essential oil) from a plant is steam distillation. The steam moves through the plant material dur...
Why is Ortho Nitrophenol steam volatile?
O-Nitrophenol forms an intramolecular H bond whereas molecules of P-Nitrophenol get associated through an intermolecular H bond. During boiling, th...
What Is the Objective of Steam Distillation?
The advantage of steam distillation over simple distillation is that the lower boiling point reduces decomposition of temperature-sensitive compounds. Steam distillation is useful for the purification of organic compounds, although vacuum distillation is more common. When organics are distilled, the vapor is condensed. Because water and organics tend to be immiscible, the resulting liquid generally consists of two phases: water and the organic distillate. Decantation or partitioning may be used to separate the two layers to obtain the purified organic material.
What happens when organics are distilled?
When organics are distilled, the vapor is condensed. Because water and organics tend to be immiscible, the resulting liquid generally consists of two phases: water and the organic distillate. Decantation or partitioning may be used to separate the two layers to obtain the purified organic material.
What are the advantages and disadvantages of changing the amount of water in a distillation mixture?
Advantages and applications: By changing amount of water in distillation mixture, the water content in steam can be adjusted.Disadvantages:Difficult to handle due to complication of apparatusProlonged contact with water causes damage to material to be distilledPresence of water in end productTime consumingMicrowave assisted steam distillation
What is the basic principle of internal steam distillation?
So, basic principle involves the interaction of steam with material to be distilled to push out volatile components from cells.
How to determine the amount of volatile acids in wine?
Amount of volatile acids in certain juices and wines is estimated by using steam distillation [14].
Why are modifications made in steam distillation apparatus?
Different modifications are made in steam distillation apparatus to get our desired results in separating different types of substances. Some of these modifications are discussed as under [5]:
Why do essential oils need to be distilled at lower temperatures?
Almost all components of essential oils are unstable at higher temperatures, so to avoid decomposition by heat, the distillation should be performed at lower temperatures and this lower temperature should be maintained.
Why are essential oils called essential oils?
Such oils were called essential because they were thought to represent the very essence of odor and flavor. Different methods of steam distillations are used for this purpose at a small scale level as well at the industrial level [13].
What temperature is used for distillation?
In this method, the desired material distilled at a temperature of fewer than 100 degrees [3].
Why do immiscible liquids boil?
The two immiscible liquids start to boil when the vapour pressure of these liquids outplace the atmospheric pressure. Many organic compounds are insoluble in water. At an absolute temperature, we can purify that is below the point where these compounds decompose.
What happens when the sum of the vapour pressures of the organic compound and water becomes equal to the atmospheric pressure?
Answer: The correct answer is option C. In steam distillation, a mixture of the organic compound and water boils at a temperature when the sum of the vapour pressures of the organic compound and water becomes equal to the atmospheric pressure. The vapour pressure of water at its boiling point is high and the organic compound will vaporize at a temperature, which is below its normal boiling point.
How does steam distillation work?
Most of the complex organic compounds do not dissolve in water, instead, they form a mixture, which separates if allowed to settle as the water settles down and the organic compounds float on top. The principle behind the steam distillation process is that when the heating of a mixture of two or more immiscible liquids ...
Why do organic compounds evaporate?
Due to the reduced vapour pressure, the required organic compounds evaporate as a part of the mixture. Moreover, its extraction takes place from organic matter. Separation Procedure- The evaporated mixture of steam and the organic compounds passes through a container that has cold water entering inside from one end.
What happens when you heat a mixture of two or more immiscible liquids?
The principle behind the steam distillation process is that when the heating of a mixture of two or more immiscible liquids takes place, the vapour pressure exerted by the system increases. This is because it now becomes the sum of the vapour pressures of all of the components of the mixture combined together.
What is the extraction process?
Extraction Procedure- In the extraction process, the steam passes through the organic matter that contains the compounds for separation. The steam condenses and forms a mixture of steam and matter.
What are the advantages of steam distillation?
Advantages of steam distillation are: 1 The method generates organic solvent-free products, 2 There is no need for subsequent separation steps, 3 Possesses large capacity for processing at the industrial scale, 4 The equipment is inexpensive, 5 Requires less fuel for the steam boiler for extraction of oils
Working and Principle of Steam Distillation
Steam Distillation works on the principle that, when two immiscible mixtures are heated together, each liquid exerts its, despite the presence of the other substance, and the vapour pressure in the system increases tremendously. This happens due to the combination of the sum of the vapour pressures of all the components of the mixture.
Steam Distillation Extraction Procedure
In the Extraction process of the compounds, a stream of strong steam is passed through the apparatus containing the organic compounds that are to be separated. If the steam is condensed then form a mixture of matter & Steam. More steam is further passed through the mixture. The mixture gets heated up and starts boiling.
Steam Distillation Separation Procedure
In the separation procedure, the mixture of steam and organic compounds that had been evaporated is passed through a container with cold water in it at one end. Further, the same mixture passes through hot water at the other end. Thus, condensation of the mixture takes place.
Process of extraction of essential oils by steam distillation
Steam distillation is an easy and efficient way to extract aromatic compounds in the form of essential oils from plants species. Steam is gently released into the apparatus and allowed to flow through the plant matter. This low-pressure steam allows to replace the volatile compounds and helps release the oil.
Applications of Steam Distillation
Steam distillation is widely used in the manufacturing of essential oils and perfumes. Mainly orange oil and eucalyptus oil are extracted using this method.
Things to Remember
Steam Distillation is used for the extraction of aromatic compounds from plant species
How is fractional distillation used?
Fractional distillation is used when the boiling points of the components of a mixture are close to each other, as determined using Raoult's law. A fractionating column is used to separate the components used a series of distillations called rectification. In fractional distillation, a mixture is heated so vapor rises and enters the fractionating column. As the vapor cools, it condenses on the packing material of the column. The heat of rising vapor causes this liquid to vaporize again, moving it along the column and eventually yielding a higher purity sample of the more volatile component of the mixture.
Why is vacuum distillation used?
Vacuum distillation is used to separate components that have high boiling points. Lowering the pressure of the apparatus also lowers boiling points. Otherwise, the process is similar to other forms of distillation. Vacuum distillation is particularly useful when the normal boiling point exceeds the decomposition temperature of a compound.
What are the different types of distillation?
Types of distillation include simple distillation, fractional distillation (different volatile 'fractions' are collected as they are produced), and destructive distillation ( usually, a material is heated so that it decomposes into compounds for collection).
What is the process of distillation called?
Although the term is most commonly applied to liquids, the reverse process can be used to separate gases by liquefying components using changes in temperature and/or pressure. A plant that performs distillation is called a distillery. The apparatus used to perform distillation is called a still .
What happens to a liquid as it cools?
As the vapor cools, it condenses on the packing material of the column. The heat of rising vapor causes this liquid to vaporize again, moving it along the column and eventually yielding a higher purity sample of the more volatile component of the mixture.
How to separate a mixture of liquids?
To separate a mixture of liquids, the liquid can be heated to force components, which have different boiling points, into the gas phase. The gas is then condensed back into liquid form and collected. Repeating the process on the collected liquid to improve the purity of the product is called double distillation.
What are some examples of distillation?
Examples of uses of distillation include purification of alcohol, desalination, crude oil refining, and making liquefied gases from air. Humans have been using distillation since at least 3000 BC in the Indus valley.