
What is Northern blot used for?
Northern Blot Definition Northern blot is a technique based on the principle of blotting for the analysis of specific RNA in a complex mixture. The technique is a modified version of the Southern Blotting, which was discovered for the analysis of DNA sequences.
What is hybridization northern blotting?
Hybridization Northern blot is a technique based on the principle of blotting for the analysis of specific RNA in a complex mixture. The technique is a modified version of the Southern Blotting, which was discovered for the analysis of DNA sequences.
What is the difference between northern and Southern blotting?
The principle is identical to southern blotting except for the probes used for the detection as northern blotting detects RNA sequences. This technique provides information about the length of the RNA sequences and the presence of variations in the sequence.
How do you identify RNA fragments in a Northern blot?
The RNA fragments are transferred out of the gel or matrix onto a solid membrane, which is then exposed to a DNA probe labeled with a radioactive, fluorescent or chemical tag. The tag allows any RNA fragments containing complementary sequences with the DNA probe sequence to be visualized within the Northern blot. … Northern blot.
What is the principle of blotting technique?
In this technique a mixture of proteins is separated based on molecular weight, and thus by type, through gel electrophoresis. These results are then transferred to a membrane producing a band for each protein. The membrane is then incubated with labels antibodies specific to the protein of interest.
What is the principle of Southern blotting?
Principle. Southern blotting is based on the principle of separation of DNA fragments by gel electrophoresis followed by the identification by labeled probe hybridization. The DNA fragments are separated based on their size and charge during electrophoresis.
What are the application of Northern blotting?
Applications. Northern blotting allows one to observe a particular gene's expression pattern between tissues, organs, developmental stages, environmental stress levels, pathogen infection, and over the course of treatment.
How do Northern blots identify RNA?
Northern blotting is a hybridization-based technique where isolated RNA is separated by gel electrophoresis, transferred to a membrane, and detected by hybridization with a DNA or RNA probe. The first detection methods involved radioactive probes.
What is the difference between Southern and Northern blotting?
Posted April 24, 2020. While both techniques are used to identify nucleic acid sequences, Northern blotting is performed to detect RNA sequences, while Southern blotting is done to detect DNA sequences. The processes for each are similar, involving gel electrophoresis, transfer to a membrane, and hybridization.
What is the difference between northern Southern and Western blotting?
The main difference between Southern Northern and Western blotting is that the Southern blotting involves the identification of DNA, and the Northern blotting involves the identification of RNA, whereas the Western blotting involves the identification of proteins.
Which gel is used in northern blotting?
Northern blot first uses denaturing gel to separate RNA according to the size. The RNA is then transferred to a nylon membrane while keeping the same distribution in the gel.
Which membrane is used in blotting?
Polyvinylidene difluoride (PVDF) membrane is ideal for western blotting applications as well as for amino acid analysis and protein sequencing of small amounts of proteins (as little as 10 pmoles). In addition, PVDF membranes can be used, stripped and reprobed without a loss of sensitivity or increased background.
Who discovered first of all northern blotting technique?
The northern blot technique was developed in 1977 by James Alwine, David Kemp and George Stank at Stanford University. The technique got its name due to the similarity of the process with Southern blotting. The primary difference between these two techniques is that northern blotting concerns only about RNA.
Does northern blotting use antibodies?
The immuno-northern blot is performed using a modified northern blotting procedure with specific antibodies against modified nucleosides.
What is the significance of northern blotting in drug discovery?
Northern blot is used to detect specific RNA sequences in a sample. Therefore it is also called the RNA blot.
Is northern blot quantitative?
However, as a quantitative technique, Northern blotting presents several limitations resulting from the inability to control for the efficiency of RNA transfer and membrane binding, as well as ill-defined factors that affect kinetics of probe hybridization to nucleic acids on a solid support.
What is application of Southern blotting?
A Southern blot is a method used in molecular biology for detection of a specific DNA sequence in DNA samples. Southern blotting combines transfer of electrophoresis-separated DNA fragments to a filter membrane and subsequent fragment detection by probe hybridization.
What is blocking in Southern blotting?
Prehybridization (Blocking): Wash the nylon membrane with a prehybridization solution containing salmon sperm DNA to block non-specific DNA interactions and reduce background noise. Alternatively, use the PerfectHyb™ Plus buffer, which doesn't require salmon sperm DNA for blocking.
What are the advantages of Southern blotting?
The advantage of this technique is that its quantitative results reflect the amounts of digested and undigested DNA molecules. Southern blot analysis is especially useful for analysis of repetitive sequences because multiple similar sequences in the genome can be analyzed by a single probe.
What is the correct sequence of events in Southern blotting?
What is the correct sequence of events in Southern blotting? Separation of DNA fragments by electrophoresis followed by transfer to a membrane and then hybridization with a labelled probe sequence.
What is Northern Blot?
Northern blot is a laboratory technique used to detect a specific RNA sequence in a blood or tissue sample. The sample RNA molecules are separated by size using gel electrophoresis. The RNA fragments are transferred out of the gel to the surface of a membrane.
Who developed the DNA protocol?
And a man named Edward Southern actually developed the protocol in which he would do a similar analysis, but you would allow DNA molecules to migrate on a gel and then transfer that a membrane. And that protocol was named after him, [and] his name is Southern.
What is the DNA probe labeled with?
The membrane is exposed to a DNA probe labeled with a radioactive or chemical tag. If the probe binds to the membrane, then the complementary RNA sequence is present in the sample.
What is the principle of Northern Blotting?
The fundamental principle of northern blotting is to separate RNA based on their size using gel electrophoresis and identified on a cellular membrane by means of hybridization probe with a base sequence corresponding to all or a part of the chain of the target RNA.
Why is northern blotting difficult?
However, there are many technical difficulties that may be encountered in northern blotting because of the process of gel fractionation of the RNA and probe preparation to variation in the quality of the blotting membrane utilized .
How is mRNA isolated from total RNA?
Some cases require the isolation of mRNA from total RNA using a poly-A+ selection procedure.The derived RNA then undergoes agarose-gel electrophoresis where it gets separated which is then proceeded by blotting onto a nylon membrane.
What is the technique used to study gene expression?
The technique that is used in molecular biology research to study gene expression by detection of RNA or isolated mRNA in a sample is called northern blotting ( RNA blotting). It is a classical method for analysis of the size and steady state level of a specific RNA in a complex sample. It is relatively simple to perform, inexpensive ...
What is the difference between Northern Blot and Northern Blot?
The major difference was the use of RNA sample to detect a specific RNA molecule within that sample. This was performed to transfercellular RNA to chemically activated cellulose paper and given the name northern blot for detection of RNA. However, the technique still made use of a radio-labelled DNA probe.
What are the different ways of isolating RNA?
Multiple ways of isolating RNA but all have some common attributes such as cellular lysis and membrane disruption, inhibiting of ribonuclease activity, deprotenization and recovery of intact RNA.
What blocks single stranded probe from binding on non-specific sites on the membrane?
Pre-hybridization blocks single stranded probe from binding on non-specific sites on the membrane.
What is Northern Blot?
A northern blot is a laboratory technique used to identify particular RNA molecules among a mixture of RNA. Northern blotting can be used to analyze a sample of RNA from a particular tissue or cell key in order to determine the RNA expression of particular genes.
How does Northern Blot work?
Northern blot first uses denaturing gel to separate RNA according to the size. The RNA is then transferred to a nylon membrane while keeping the exact same distribution in the gel. After fixing the RNA to the membrane, a labeled probe complementary to the gene of interest is then added to hybridize to the debilitated RNA.
What gel is used for RNA separation?
The RNA sequence is separated in the electrophoresis system an agarose gel is used for the function of the nucleic acid separation.
Why is the blot membrane washed?
The blot membrane is washed to get rid of unwanted probes.
Why is RNA degraded by RNase?
RNA is slightly degraded by RNase due to contamination by water, contaminated hands, or tips and equipment can negatively affect the quantitation.
When was Northern Blot developed?
The northern blot method was developed in 1977 by James Alwin, David Kemp, and George Stark at Stanford University, with contributions from Gerhard Heinrich. Northern blotting takes its name from its similarity to the first blotting technique, the Southern blot.
Does Northern Blot use size dependent separation?
Given that northern blot uses size-dependent separation, this strategy can not only figure out the abundance but likewise the sizes of a transcript of interest.
How does Northern Blotting work?
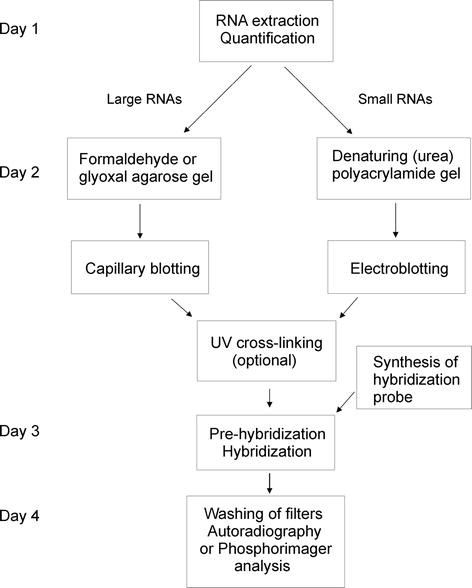
As all normal blotting technique, northern blotting starts with the electrophoresis to separate RNA samples by size. Electrophoresis separates the RNA molecules based on the charge of the nucleic acids. The charge in the nucleic acids is proportional to the size of the nucleic acid sequence. Thus the electrophoresis membrane separates the Nucleic acid sequence according to the size of the RNA sequence. In cases where our target sequence is an mRNA, the sample can be isolated through oligo cellulose chromatographic techniques, as mRNA are characterized by the poly (A)-tail. Since gel molecules are fragile in nature, the separated sequences are transferred to the nylon membranes. The selection of nylon membrane is contributed to the factor that nucleic acids are negatively charged in nature. Once the RNA molecules are transferred it is immobilized by covalent linkage. The probe is then added, the probe can be complementary an ss DNA sequence. Formamide is generally used as a blotting buffer as it reduces the annealing temperature.
When was Northern Blot developed?
The northern blot technique was developed in 1977 by James Alwine, David Kemp and George Stank at Stanford University. The technique got its name due to the similarity of the process with Southern blotting. The primary difference between these two techniques is that northern blotting concerns only about RNA.
How is blotting done?
It is done by detection of particular RNA (or isolated mRNA). mRNA is generally represented as 5% of the overall RNA sequence. This method reveals the identity, number, activity, and size of the particular gene. This blotting technique can also be used for the growth of a tissue or organism.
What is blotting used for?
Blotting is used in molecular biology for the identification of proteins and nucleic acids and is widely used for diagnostic purposes. This technique immobilizes the molecule of interest on a support, which is a nitrocellulosic membrane or nylon. It uses hybridization techniques for the identification of the specific nucleic acids and genes.
What is used to separate RNA samples?
The RNA samples are separated using agarose gels using formaldehyde as denaturing agents but in small RNA or micro RNA sequences, polyacrylamide sequences with urea as a denaturing agent also can be used. Ethidium bromide can be used as a staining agent. Two types of markers are for size marking.
How is separated RNA transferred to the nylon membrane?
Now the separated RNA sequence is transferred to the nylon membrane. This is done by two mechanisms capillary action and the ionic interaction.
What is the purpose of blotting?
The blotting technique is a tool used in the identification of biomolecules such ad DNA, mRNA and protein during different stages of gene expression. Protein synthesis involves expression of a DNA segment which gets converted to mRNA to produce the respective protein.
How does Northern Blotting work?
Northern Blotting: Principle, Procedure and Applications 1 The reactions are specific-the probes will only bind to targets with a complementary sequence. 2 The probe can find one molecule of target in a mixture of millions of related but non-complementary molecules.
What is Northern Blot?
The Northern blot is used to detect the presence of a particular mRNA in a sample. The term “Northern” has no scientific significance just a misnomer. Principle: The key to this method is hybridization. Hybridization: It is the process of forming a double-stranded DNA-RNA hybrid molecule between a single-stranded DNA probe ...
What is the process of forming a double stranded DNA-RNA hybrid molecule between a single?
Hybridization: It is the process of forming a double-stranded DNA-RNA hybrid molecule between a single-stranded DNA probe and a single-stranded target RNA. There are 2 important features of hybridization: The reactions are specific-the probes will only bind to targets with a complementary sequence.
What is the transfer of RNA from gel to membrane called?
4. Transfer to aminobenzyloxymethyl filter paper. The transfer of RNA from gel to membrane is called blotting
What is Northern Blot?
Northern blot is a technique based on the principle of blotting for the analysis of specific RNA in a complex mixture.
What is Northern Blotting used for?
Northern blotting has been used as a molecular diagnostic tool for diseases such as Crohn’s disease.
How is Northern Blot made?
The northern blot for RNA detection was created by transferring cellular RNA to chemically activated cellulose paper. However, a radio-labelled DNA probe was still used in the procedure.
How to prevent nucleic acid from washing away?
To prevent nucleic acid from washing away later, the RNA on the membrane must be immobilised by baking or exposure to UV light after blotting.
What is the agent that dissolves RNA?
To begin, we extract RNA from a tissue with chaotropic agents like guanidinium isothiocynate, which disrupts cells and denaturates proteins while also dissolving the RNA.
What are the characteristics of isolating RNA?
There are several methods for isolating RNA, but they all share some characteristics, such as cellular lysis and membrane disruption, inhibition of ribonuclease activity, deproteinization, and recovery of intact RNA.
How long does it take to incubate a RNA sample?
For about 12-15 minutes, the samples are incubated at 65°C on a heating block. The samples are loaded into the equilibrated gel, and the first row of wells is populated with RNA markers. After that, the gel is run at 125V for about 3 hours.

History
- The trend set by Southern blotting (in 1975) to detect specific DNA brought new ideas in the field of modern molecular biology. The method got modified in 1977, to develop something very similar to the southern blot when James Alwin, David Kemp and George Stark at Stanford University repeated the design of the southern blot. The major difference was the use of RNA sample to de…
Definition
- The technique that is used in molecular biology research to study gene expression by detection of RNA or isolated mRNA in a sample is called northern blotting (RNA blotting). It is a classical method for analysis of the size and steady state level of a specific RNA in a complex sample. It is relatively simple to perform, inexpensive and not obstructed by artefacts. However, there are ma…
Principle
- The fundamental principle of northern blotting is to separate RNA based on their size using gel electrophoresis and identified on a cellular membrane by means of hybridization probe with a base sequence corresponding to all or a part of the chain of the target RNA. Initially, we extract RNA from a tissue through chaotropic agents such as guanidiniu...
Procedure
- RNA Isolation
- Multiple ways of isolating RNA but all have some common attributes such as cellular lysis and membrane disruption, inhibiting of ribonuclease activity, deprotenization and recovery of intact RNA.
- Some of the common methods of RNA extraction are:
- RNA Isolation
- Multiple ways of isolating RNA but all have some common attributes such as cellular lysis and membrane disruption, inhibiting of ribonuclease activity, deprotenization and recovery of intact RNA.
- Some of the common methods of RNA extraction are:
- Assessment of quantity and quality of RNA through spectrophotometry.
Applications
- Standard for studying and inspecting gene manifestationdesign between tissues, organs, developmental stages, pathogen infection, and over the course of treatment.
- Diagnosis of several diseases (Crohn’s diseases) including viral infection.
- Exhibit overexpression of oncogenes and down regulation of tumor suppressor genes in cancerous cells.
- Standard for studying and inspecting gene manifestationdesign between tissues, organs, developmental stages, pathogen infection, and over the course of treatment.
- Diagnosis of several diseases (Crohn’s diseases) including viral infection.
- Exhibit overexpression of oncogenes and down regulation of tumor suppressor genes in cancerous cells.
- Study of RNA degradation and splicing.
Advantage and Disadvantages
- Advantages 1. Relatively simple, cost effective, reduced artifacts. 2. Spotted membranes can be stripped of the probes and are reused for hybridization. 3. Detect slightest of gene expression changes due to its sensitivity. 4. Due to dilution and housekeeping genes control, the results are highly reliable. Disadvantages 1. Time consuming (Only one gene can be analyzed at a time). 2…
References
- Kenneth M.Rosen, Edward D. Lamperti, Lydia Villa-Komaroff (1990), Optimizing the Northern Blot Procedure
- Paul Trayhurn (1996), Northern blotting
- Akhira Muto, Ken-ichi Arai (1998), Northern Blotting
- Terry Brown, Karol Mackey, Tingting Du (2004), Analysis of RNA by Northern and Slot Blot H…
- Kenneth M.Rosen, Edward D. Lamperti, Lydia Villa-Komaroff (1990), Optimizing the Northern Blot Procedure
- Paul Trayhurn (1996), Northern blotting
- Akhira Muto, Ken-ichi Arai (1998), Northern Blotting
- Terry Brown, Karol Mackey, Tingting Du (2004), Analysis of RNA by Northern and Slot Blot Hybridization
Brief Introduction: Blotting Techniques
Northern Blotting
- Northern Blotting is a technique used for the study of gene expression. It is done by detection of particular RNA (or isolated mRNA). mRNA is generally represented as 5% of the overall RNA sequence. This method reveals the identity, number, activity, and size of the particular gene. This blotting technique can also be used for the growth of a tissue or organism. In different stages of …
Principle
- As all normal blotting technique, northern blotting starts with the electrophoresis to separate RNA samples by size. Electrophoresis separates the RNA molecules based on the charge of the nucleic acids. The charge in the nucleic acids is proportional to the size of the nucleic acid sequence. Thus the electrophoresis membrane separates the Nucleic a...
Procedure
- The tissue or culture sample collected is first homogenized. The samples may be representative of different types of culture for comparison or it can be for the study of different stages of growth...
- The RNA sequence is separated in the electrophoresis unit an agarose gel is used for the purpose of the nucleic acid separation.
- The tissue or culture sample collected is first homogenized. The samples may be representative of different types of culture for comparison or it can be for the study of different stages of growth...
- The RNA sequence is separated in the electrophoresis unit an agarose gel is used for the purpose of the nucleic acid separation.
- Now the separated RNA sequence is transferred to the nylon membrane. This is done by two mechanisms capillary action and the ionic interaction.
- The transfer operation is done by keeping the gel in the following order. First, the agarose gel is placed on the bottom of the stack, followed by the blotting membrane. On top of these paper towel...
Gel and Probes
- The RNA samples are separated using agarose gels using formaldehyde as denaturing agents but in small RNA or micro RNA sequences, polyacrylamide sequences with urea as a denaturing agent also can be used. Ethidium bromide can be used as a staining agent. Two types of markers are for size marking. An RNA ladder and ribosomal subunit are used for the identification of the …