Which structure is the site of the synthesis of proteins that may be exported from the cell?
- Endoplasmic reticulum. Proteins are synthesized at ribosomes. ...
- Rough endoplasmic reticulum. The RER consists of a series of sacs known as cisternae, surrounding a space known as the lumen. ...
- Golgi apparatus. This is an organelle that is closely associated with the RER in the cell. ...
- Example of prohormone formation and transport. ...
- References. ...
How are proteins made in a cell?
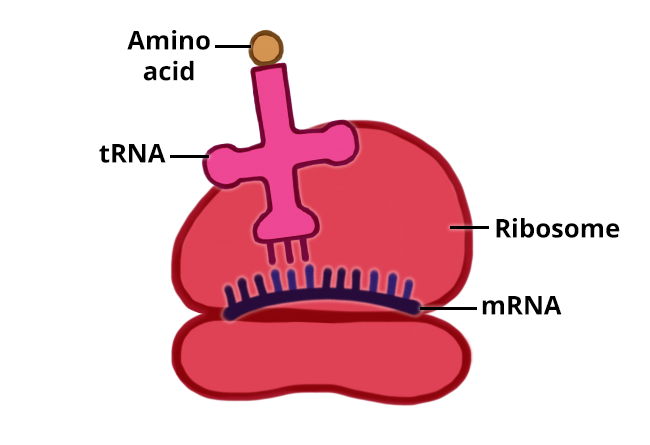
The information to produce a protein is encoded in the cell’s DNA. When a protein is produced, a copy of the DNA is made (called mRNA) and this copy is transported to a ribosome. Ribosomes read the information in the mRNA and use that information to assemble amino acids into a protein.
Where does protein synthesis occur in a cell?
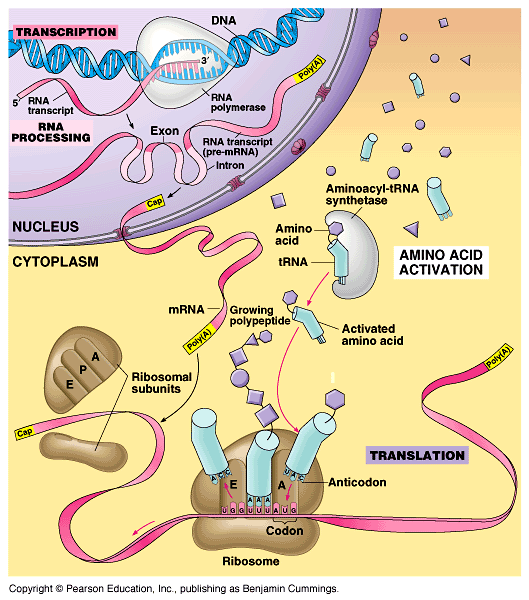
Protein synthesis takes place in the cytopladm outside the cell´s nucleus. The chromosomes are found inside the nucleus, so a messenger must carry the genetic code from the DNA inside the nucleusto the cytoplasm. This genetic code messenger is called RNA, or ribonucleic acid.
Where are the protein making factories located in the cell?
However, the protein making factories (ribosomes) are located in the cytoplasm outside of the nucleus. How does the cell solve this problem? It must send a “messenger” that carries a copy of the genetic information from the nucleus to the ribosomes in the cytoplasm.
Where is the information to produce proteins encoded?
The information to produce a protein is encoded in the cell’s DNA. When a protein is produced, a copy of the DNA is made (called mRNA) and this copy is transported to a ribosome.
Where does mRNA go in the cell?
The molecule of mRNA then stops the nucleus and goes to a ribosome in the cytoplasm, where translation materializes. Through translation, the genetic code in mRNA is analyzed and used to create a protein. Related – How Much Does a Gallon of Water Weigh?
What are the two processes that make up protein synthesis?
Protein synthesis really consists of two processes — translation and transcription
What are the four macromolecules found in living organisms?
Protein is just one of four macromolecules found in living organisms. Macromolecules are enormous molecules that serve a collection of functions inside living organisms. The other three kinds of macromolecules are carbohydrates, lipids (fats), and nucleic acids like DNA, and much enjoy this, protein plays with various purposes. But before we talk about those purposes, let us have a more solid look at the way this particular protein is designed, to start.
What are the regions that do not code for a protein?
Introns are regions that do not code for the protein. The remaining mRNA consists just of areas called exons that do code for the protein. The ribonucleoproteins from the diagram are small proteins in the nucleus that contain RNA and are needed for the splicing process.
What is DNA used for?
Inside each cell, catalysts pursue out the proper education from this archive and use it to build new proteins that compose the arrangements of the mobile, run the biochemical reactions in the cell, and are sometimes built for export.
What is the tail of mRNA?
Polyadenylation adds a”tail” to the mRNA. The tail includes a series of As (adenine bases). It signals the end of mRNA. It is also engaged in exporting mRNA in the nucleus, and it secures mRNA from enzymes that might damage it down.
Do all cells in a multicellular organism have the same genetic information?
Although every one of the cells that make up a multicellular organism incorporates identical genetic information, functionally distinct cells within the organism use disparate sets of catalysts to express only specific servings of those instructions to perform the functions of existence.
Where is DNA stored in eukaryotic cells?
Now consider the location of Eukaryotic DNA. Eukaryotic organisms protect their DNA by storing it inside the nucleus. However, the protein making factories (ribosomes) are located in the cytoplasm outside of the nucleus. How does the cell solve this problem? It must send a “messenger” that carries a copy of the genetic information from the nucleus to the ribosomes in the cytoplasm.
What are the two processes required for protein synthesis?
Protein synthesis requires two distinct processes, transcription and translation . You will have an opportunity to review both procedures as you “make a protein.”. You will use your textbook and the information in this lab as a reference.
What is the result of transcription?
Transcription results in the formation of an mRNA molecule that carries the instructions for the specific protein to the ribosome where the information is “translated” into a sequence of amino acids to form a protein.
What is the process of transferring DNA from the nucleus to the ribosomes?
It must send a “messenger” that carries a copy of the genetic information from the nucleus to the ribosomes in the cytoplasm. Protein synthesis is a two-step process that involves two main events called transcription and translation. In transcription, the DNA code is transcribed (copied) into mRNA.
How many amino acids are in a DNA word?
In DNA code, a “word” is always 3 letters long and it specifies one of 20 amino acids. However, DNA is not directly involved in the translation process, instead mRNA is transcribed into a sequence of amino acids. When reading the mRNA, it is “read” in a series of three adjacent nucleotides.
What is the start signal of globin?
Methionine is the “Start” signal. Write its codon in the space provided. Globin is a red blood cell protein that is responsible for oxygen transport. The amino acid sequence for a portion of the globin protein is Proline, Glutamic Acid, Glutamic Acid, Lysine.
How is mRNA read?
When reading the mRNA, it is “read” in a series of three adjacent nucleotides. In transcription, the DNA code is transcribed (copied) into RNA code, following rules similar to DNA replication we saw earlier except that Thymine (T) is replaced by Uracil (U). DNA. RNA.
Where does protein synthesis take place?
Protein synthesis takes place in the cytopladm outside the cell´s nucleus. The chromosomes are found inside the nucleus, so a messenger must carry the genetic code from the DNA inside the nucleusto the cytoplasm. This genetic code messenger is called RNA, or ribonucleic acid.
What happens during protein synthesis?
During protein synthesis, the cell uses information from a gene on a chromosome to produce a specific protein.
How do amino acids form?
The order of the amino acids is determines by the order of the three-base codes in the mRNA. Step 5: Protein Chains Forms: As the ribosome continues to move along the mRNA adding amino acids, the proteins grows.
What is the process of mRNA leaving the nucleus and entering the cytoplasm?
The process is similar to DNA replication. In this step the mRNA leaves the nucleus and enters the cytoplasm. Step 2: Ribosomes attach to mRNA: A ribosome attaches to mRNA in the cytoplasm. On the ribosome, the mRNA provides the code for the protein that will be made.
How many amino acids are in a protein?
The structure of proteins. Proteins are made up of molecules called amino acids. Altough there are only 20 amino acids, cells can combine them in different ways to from thousands of different proteins.
Where is the protein that is used in the cell?
If the protein is going to be used within the cytoplasm of the cell, the ribosome creating the protein will be free-floating in the cytoplasm. If the protein is going to be targeted to the lysosome, become a component of the plasma membrane, or be secreted outside of the cell, the protein will be synthesized by a ribosome located on ...
Where is the information that makes a protein?
The information to produce a protein is encoded in the cell’s DNA. When a protein is produced, a copy of the DNA is made (called mRNA) and this copy is transported to a ribosome. Ribosomes read the information in the mRNA and use that information to assemble amino acids into a protein. If the protein is going to be used within the cytoplasm of the cell, the ribosome creating the protein will be free-floating in the cytoplasm. If the protein is going to be targeted to the lysosome, become a component of the plasma membrane, or be secreted outside of the cell, the protein will be synthesized by a ribosome located on the rough endoplasmic reticulum (RER). After being synthesized, the protein will be carried in a vesicle from the RER to the cis face of the Golgi (the side facing the inside of the cell). As the protein moves through the Golgi, it can be modified. Once the final modified protein has been completed, it exits the Golgi in a vesicle that buds from the trans face. From there, the vesicle can be targeted to a lysosome or targeted to the plasma membrane. If the vesicle fuses with the plasma membrane, the protein will become part of the membrane or be ejected from the cell.
What happens when a vesicle fuses with the plasma membrane?
If the vesicle fuses with the plasma membrane, the protein will become part of the membrane or be ejected from the cell. Figure 3 Diagram of a eukaryotic cell.
Why are proteins so diverse?
The functions of proteins are very diverse because they are made up of are 20 different chemically distinct amino acids that form long chains, and the amino acids can be in any order. The function of the protein is dependent on the protein’s shape. The shape of a protein is determined by the order of the amino acids.
How is the shape of a protein determined?
The shape of a protein is determined by the order of the amino acids. Proteins are often hundreds of amino acids long and they can have very complex shapes because there are so many different possible orders for the 20 amino acids! Figure 1 Protein structure.
What hormone is produced by beta cells?
Insulin. Insulin is a protein hormone that is made by specific cells inside the pancreas called beta cells. When the beta cells sense that glucose (sugar) levels in the bloodstream are high, they produce insulin protein and secrete it outside of the cells into the bloodstream.
Where does a protein go after being synthesized?
After being synthesized, the protein will be carried in a vesicle from the RER to the cis face of the Golgi (the side facing the inside of the cell). As the protein moves through the Golgi, it can be modified. Once the final modified protein has been completed, it exits the Golgi in a vesicle that buds from the trans face.
What is ribosome binding site?
Ribosome binding sites are short RNA sequences upstream of a gene's coding sequence. In the past, biotechnologists also developed synthetic binding sites. The ribosomes bind extremely well to some of these, and less well to others. The tighter ribosomes are able to bind to a specific variant, the more often they translate the respective gene and the greater the amount of the corresponding protein they produce.
How do bacteria influence proteins?
Biotechnologists who use bacteria to produce chemicals of interest such as pharmaceuticals can influence the amount of involved proteins in the cell through their choice of ribosome binding sites. "Exerting this kind of control is particularly important and helpful when incorporating complex gene networks comprising multiple proteins at the same time. The key here is to establish an optimal balance amongst the different proteins," says Markus Jeschek, senior scientist and group leader at D-BSSE.
Who developed the method to determine how tightly ribosomes bind to hundreds of thousands or more RNA sequence?
Together with ETH professors Yaakov Benenson and Karsten Borgwardt and members of the respective groups, Jeschek has now developed a method to determine how tightly ribosomes bind to hundreds of thousands or more RNA sequences in a single experiment. Previously this was only possible for a few hundred sequences.
What is the function of ribosomes?
Ribosomes are the cell's protein-making machinery. They read the genetic information encoded in messenger RNA (violet) and translate it into proteins (yellow). Credit: Science Photo Library
What is the binding site of a protein?
The binding site of a protein. (A) The folding of the polypeptide chain typically creates a crevice or cavity on the protein surface. This crevice contains a set of amino acid side chains disposed in such a way that they can make noncovalent bonds only (more...)
How do proteins work?
Many proteins can perform their function simply by binding to another molecule. An actin molecule, for example, need only associate with other actin molecules to form a filament. There are other proteins, however, for which ligand binding is only a necessary first step in their function. This is the case for the large and very important class of proteins called enzymes. As described in Chapter 2, enzymes are remarkable molecules that determine all the chemical transformations that make and break covalent bonds in cells. They bind to one or more ligands, called substrates, and convert them into one or more chemically modified products, doing this over and over again with amazing rapidity. Enzymes speed up reactions, often by a factor of a million or more, without themselves being changed—that is, they act as catalysts that permit cells to make or break covalent bonds in a controlled way. It is the catalysis of organized sets of chemical reactions by enzymes that creates and maintains the cell, making life possible.
How are protein kinases organized in eucaryotic cells?
The hundreds of different protein kinases in a eucaryotic cell are organized into complex networks of signaling pathways that help to coordinate the cell’s activities, drive the cell cycle, and relay signals into the cell from the cell’s environment.
How do ligands affect proteins?
The effects of ligand binding on a protein follow from a fundamental chemical principle known as linkage. Suppose, for example, that a protein that binds glucose also binds another molecule, X, at a distant site on the protein’s surface. If the binding site for X changes shape as part of the conformational change induced by glucose binding, the binding sites for X and for glucose are said to be coupled. Whenever two ligands prefer to bind to the same conformation of an allosteric protein, it follows from basic thermodynamic principles that each ligand must increase the affinity of the protein for the other. Thus, if the shift of the protein in Figure 3-57 to the closed conformation that binds glucose best also causes the binding site for X to fit X better, then the protein will bind glucose more tightly when X is present than when X is absent.
How do enzymes catalyze chemical reactions?
To demonstrate how enzymes catalyze chemical reactions, we shall use the example of an enzyme that acts as a natural antibiotic in egg white, saliva, tears, and other secretions. Lysozyme is an enzyme that catalyzes the cutting of polysaccharide chains in the cell walls of bacteria. Because the bacterial cell is under pressure from osmotic forces, cutting even a small number of polysaccharide chains causes the cell wall to rupture and the cell to burst. Lysozyme is a relatively small and stable protein that can be easily isolated in large quantities. For these reasons, it has been intensively studied, and it was the first enzyme to have its structure worked out in atomic detail by x-ray crystallography.
What is the ability of a protein to bind selectively and with high affinity to a ligand?
The ability of a protein to bind selectively and with high affinity to a ligand depend s on the formation of a set of weak, noncovalent bonds—hydrogen bonds, ionic bonds, and van der Waals attractions—plus favorable hydrophobic interactions (see Panel 2-3, pp. 114–115).
What is the selective binding of a protein to another molecule?
The selective binding of a protein to another molecule. Many weak bonds are needed to enable a protein to bind tightly to a second molecule, which is called a ligand for the protein. A ligand must therefore fit precisely into a protein’s binding (more...)
Where is protein synthesis performed?
To maintain the correct reading frame and to ensure accuracy (about 1 mistake every 10,000 amino acids), protein synthesis is performed in the ribosome, a complex catalytic machine made from more than 50 different proteins (the ribosomal proteins) and several RNA molecules, the ribosomal RNAs (rRNAs). A typical eucaryotic cell contains millions of ribosomes in its cytoplasm ( Figure 6-62 ). As we have seen, eucaryotic ribosomal subunits are assembled at the nucleolus, by the association of newly transcribed and modified rRNAs with ribosomal proteins, which have been transported into the nucleus after their synthesis in the cytoplasm. The two ribosomal subunits are then exported to the cytoplasm, where they perform protein synthesis.
How long does it take for a protein to be synthesized?
The synthesis of most protein molecules takes between 20 seconds and several minutes. But even during this very short period, multiple initiations usually take place on each mRNA molecule being translated. As soon as the preceding ribosome has translated enough of the nucleotide sequence to move out of the way, the 5′ end of the mRNA is threaded into a new ribosome. The mRNA molecules being translated are therefore usually found in the form of polyribosomes (also known as polysomes ), large cytoplasmic assemblies made up of several ribosomes spaced as close as 80 nucleotides apart along a single mRNA molecule ( Figure 6-75 ). These multiple initiations mean that many more protein molecules can be made in a given time than would be possible if each had to be completed before the next could start.
What is the end of a protein coding message?
The end of the protein -coding message is signaled by the presence of one of three codons (UAA, UAG, or UGA) called stop codons (see Figure 6-50 ). These are not recognized by a tRNA and do not specify an amino acid, but instead signal to the ribosome to stop translation. Proteins known as release factors bind to any ribosome with a stop codon positioned in the A site, and this binding forces the peptidyl transferase in the ribosome to catalyze the addition of a water molecule instead of an amino acid to the peptidyl-tRNA ( Figure 6-73 ). This reaction frees the carboxyl end of the growing polypeptide chain from its attachment to a tRNA molecule, and since only this attachment normally holds the growing polypeptide to the ribosome, the completed protein chain is immediately released into the cytoplasm. The ribosome then releases the mRNA and separates into the large and small subunits, which can assemble on another mRNA molecule to begin a new round of protein synthesis.
How does tRNA synthetase work?
Several mechanisms working together ensure that the tRNA synthetase links the correct amino acid to each tRNA. The synthetase must first select the correct amino acid, and most do so by a two-step mechanism. First, the correct amino acid has the highest affinity for the active-site pocket of its synthetase and is therefore favored over the other 19. In particular, amino acids larger than the correct one are effectively excluded from the active site. However, accurate discrimination between two similar amino acids, such as isoleucine and valine (which differ by only a methyl group), is very difficult to achieve by a one-step recognition mechanism. A second discrimination step occurs after the amino acid has been covalently linked to AMP (see Figure 6-56 ). When tRNA binds the synthetase, it forces the amino acid into a second pocket in the synthetase, the precise dimensions of which exclude the correct amino acid but allow access by closely related amino acids. Once an amino acid enters this editing pocket, it is hydrolyzed from the AMP (or from the tRNA itself if the aminoacyl-tRNA bond has already formed) and released from the enzyme. This hydrolytic editing, which is analogous to the editing by DNA polymerases ( Figure 6-59 ), raises the overall accuracy of tRNA charging to approximately one mistake in 40,000 couplings.
What is the ribosome made of?
The ribosome is a very large and complex structure, composed of two-thirds RNA and one-third protein. The determination, in 2000, of the entire three-dimensional structure of its large and small subunits is a major triumph of modern structural biology. The structure strongly confirms the earlier evidence that rRNAs—and not proteins—are responsible for the ribosome's overall structure, its ability to position tRNAs on the mRNA, and its catalytic activity in forming covalent peptide bonds. Thus, for example, the ribosomal RNAs are folded into highly compact, precise three-dimensional structures that form the compact core of the ribosome and thereby determine its overall shape ( Figure 6-67 ).
What is the final phase of protein synthesis?
The final phase of protein synthesis. The binding of a release factor to an A-site bearing a stop codon terminates translation. The completed polypeptide is released and, after the action of a ribosome recycling factor (not shown), the ribosome dissociates (more...)
What is the final product of genes?
In the preceding section we have seen that the final product of some genes is an RNA molecule itself, such as those present in the snRNPs and in ribosomes. However, most genes in a cell produce mRNA molecules that serve as intermediaries on the pathway to proteins. In this section we examine how the cell converts the information carried in an mRNA molecule into a protein molecule. This feat of translation first attracted the attention of biologists in the late 1950s, when it was posed as the “coding problem”: how is the information in a linear sequence of nucleotides in RNA translated into the linear sequence of a chemically quite different set of subunits—the amino acids in proteins? This fascinating question stimulated great excitement among scientists at the time. Here was a cryptogram set up by nature that, after more than 3 billion years of evolution, could finally be solved by one of the products of evolution—human beings. And indeed, not only has the code been cracked step by step, but in the year 2000 the elaborate machinery by which cells read this code—the ribosome —was finally revealed in atomic detail.
Which proteins provide structure and support for cells?
Growth hormone. Structural component. These proteins provide structure and support for cells. On a larger scale, they also allow the body to move. Actin. Transport/storage. These proteins bind and carry atoms and small molecules within cells and throughout the body. Ferritin.
What do proteins do?
Proteins are large, complex molecules that play many critical roles in the body. They do most of the work in cells and are required for the structure, function, and regulation of the body’s tissues and organs.
What determines the sequence of amino acids?
The sequence of amino acids determines each protein’s unique 3-dimensional structure and its specific function. Amino acids are coded by combinations of three DNA building blocks (nucleotides), determined by the sequence of genes.
Where is the genetics home reference?
Genetics Home Reference has merged with MedlinePlus. Genetics Home Reference content now can be found in the "Genetics" section of MedlinePlus. Learn more
Why do antibodies bind to specific foreign particles?
Antibodies bind to specific foreign particles, such as viruses and bacteria, to help protect the body.
How do proteins fold?
Most proteins fold into unique 3D structures. The shape into which a protein naturally folds is known as its native conformation. Although many proteins can fold unassisted, simply through the chemical properties of their amino acids, others require the aid of molecular chaperones to fold into their native states. Biochemists often refer to four distinct aspects of a protein's structure: 1 Primary structure: the amino acid sequence. A protein is a polyamide. 2 Secondary structure: regularly repeating local structures stabilized by hydrogen bonds. The most common examples are the α-helix, β-sheet and turns. Because secondary structures are local, many regions of different secondary structure can be present in the same protein molecule. 3 Tertiary structure: the overall shape of a single protein molecule; the spatial relationship of the secondary structures to one another. Tertiary structure is generally stabilized by nonlocal interactions, most commonly the formation of a hydrophobic core, but also through salt bridges, hydrogen bonds, disulfide bonds, and even posttranslational modifications. The term "tertiary structure" is often used as synonymous with the term fold. The tertiary structure is what controls the basic function of the protein. 4 Quaternary structure: the structure formed by several protein molecules (polypeptide chains), usually called protein subunits in this context, which function as a single protein complex. 5 Quinary structure: the signatures of protein surface that organize the crowded cellular interior. Quinary structure is dependent on transient, yet essential, macromolecular interactions that occur inside living cells.
What are the structural features of proteins?
All proteinogenic amino acids possess common structural features, including an α-carbon to which an amino group, a carboxyl group, and a variable side chain are bonded . Only proline differs from this basic structure as it contains an unusual ring to the N-end amine group, which forces the CO–NH amide moiety into a fixed conformation. The side chains of the standard amino acids, detailed in the list of standard amino acids, have a great variety of chemical structures and properties; it is the combined effect of all of the amino acid side chains in a protein that ultimately determines its three-dimensional structure and its chemical reactivity. The amino acids in a polypeptide chain are linked by peptide bonds. Once linked in the protein chain, an individual amino acid is called a residue, and the linked series of carbon, nitrogen, and oxygen atoms are known as the main chain or protein backbone. : 19
How many proteins are encoded in a genome?
The number of proteins encoded in a genome roughly corresponds to the number of genes (although there may be a significant number of genes that encode RNA of protein, e.g. ribosomal RNAs ). Viruses typically encode a few to a few hundred proteins, archaea and bacteria a few hundred to a few thousand, while eukaryotes typically encode a few thousand up to tens of thousands of proteins (see genome size for a list of examples).
How long do proteins live?
A protein's lifespan is measured in terms of its half-life and covers a wide range. They can exist for minutes or years with an average lifespan of 1–2 days in mammalian cells. Abnormal or misfolded proteins are degraded more rapidly either due to being targeted for destruction or due to being unstable.
How do proteins differ from each other?
Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide.
What is the name of the chain of amino acids?
The amino acids in a polypeptide chain are linked by peptide bonds. Once linked in the protein chain, an individual amino acid is called a residue, and the linked series of carbon, nitrogen, and oxygen atoms are known as the main chain or protein backbone.
How are amino acids bonded together?
The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residues in a protein is defined by the sequence of a gene, which is encoded in the genetic code.
