A Catalyst and the Rate of Reaction
- Key Concepts. A catalyst is a substance that can help the reactants in a chemical reaction react with each other faster.
- Summary. ...
- Objective. ...
- Evaluation. ...
- Safety. ...
- Materials for Each Group
- Notes about the Materials. ...
What are some examples of reactions that involve catalysts?
- Catalytic oxidation of lower alkanes i.e CH4 + [O] is converted into methyl alcohol in presence of copper as a catalyst
- Partial hydrogenation of alkynes takes place in the presence of lindlar's catalyst [pd (BaSO4)]/quinoline i.e. ...
- Addition of ammonia to ethyne takes place in the presence of Al2O3.
What is the main function of a catalyst?
What is the main function of a catalyst? A catalyst is a substance that is used to speed up a chemical reaction but is not consumed by the reaction, so it can be recovered at the end of the reaction. Let's Get It Fixed!
What is the purpose of a catalyst?
Suitable chemical catalysts are used to promote and speed up the polymerization process—in which the individual building blocks connect to form large polymer molecules.
What are the types of catalysts?
- Homogeneous catalysis
- Heterogeneous catalysis
- Autocatalysis

What is the Purpose of a Catalyst?
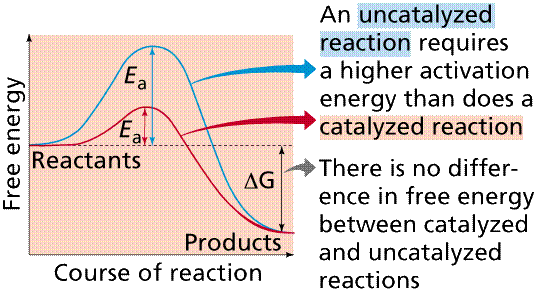
As previously mentioned, the function of catalysts is to speed up the rate of a reaction without being consumed in the process. Therefore, it is not classified as either a reactant or product in the reaction. The specific process by which catalysts speed up a reaction is by lowering the activation energy of the reaction - which is the energy state required to start off the reaction.
What is catalyst in chemistry?
What is a catalyst? A catalyst is a term used in chemistry to describe substances that can speed up a reaction, without being used up in the process. The catalyst definition also states that they that can change the conditions required for a reaction to processes, such as the required temperature or pressure.
What is a homogeneous catalyst?
Homogeneous catalysts are catalysts that occupy the same phase as the reactants they are interacting with. This could either mean that the catalyst is of the same state of matter (solid, liquid, or gas) as the reactants and well mixed, or that it can dissolve into a solution with the reactants.
How does a catalyst work?
Catalysts work by lowering the activation energy - i.e., the energy state required to start a reaction - by forming temporary, weak bonds with the reactant molecules. By the end of the reaction, however, these bonds are lost and the catalyst returns to its original state, which is why no net change occurs to the catalyst. Many well-known examples of catalysts are enzymes, which are biological catalysts found in living organisms.
Why do reactants form stronger bonds?
Because these bonds are weak, the molecules of one reactant bond to the molecules of the other reactant when they collide, while bonded to the surface of the catalyst. This forms the reaction product, which has stronger and more permanent bonds. In this process, they separate from the catalyst, and the catalyst returns to its original state, unconsumed and unchanged by the reaction.
What are some examples of catalysts?
A very common example of catalysts that are important in our everyday lives are the enzymes found in our body (as well as, in other living organisms.) These enzymes are biological catalysts that speed up biochemical reactions inside the body. They carry out catalysis using a lock and key model, in which the reactants (called substrates) fit perfectly into the shape of the enzyme. An example of an enzyme is Pepsin, which is secreted by the stomach and catalyzes the breakdown of the proteins that are consumed, as part of digestion.
What is the metal in a car that converts carbon monoxide to carbon dioxide?
Catalytic converters, found in cars, contain the metal platinum which catalyzes the conversion of toxic carbon monoxide gas into carbon dioxide.
What is a catalyst?
A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, ...
Why are catalysts important?
Using catalysts leads to faster, more energy-efficient chemical reactions. Catalysts also have a key property called selectivity, by which they can direct a reaction to increase the amount of desired product and reduce the amount of unwanted byproducts. They can produce entirely new materials with entirely new potential uses.
How do catalysts make a chemical reaction more efficient?
Catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be surmounted for a chemical reaction to occur.
What is the process of adding a catalyst to a chemical reaction?
A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself being consumed during the reaction. Catalysis is the process of adding a catalyst to facilitate a reaction.
What are catalysts in bread?
Fast Facts. Humans have been using catalysts for thousands of years. For example, the yeast we use to make bread contains enzymes, which are natural catalysts that aid the conversion of flour into bread.