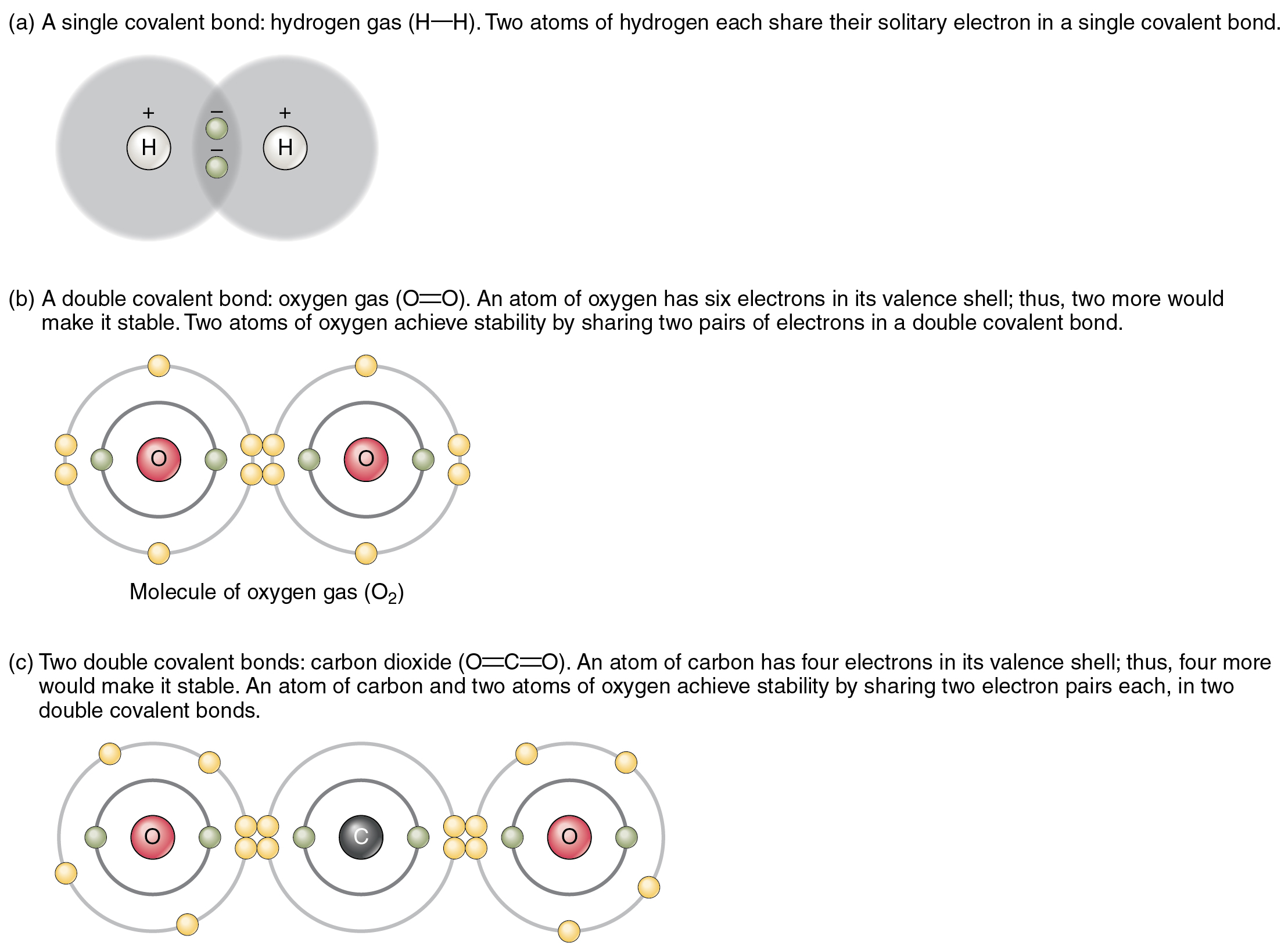
An element's valency is determined by the number of electrons in its outer shell. Hence, the number of valence electrons obtained from the electronic configuration of the element gives the valency i.e. the number of electrons lost, gained or shared by the element to attain the noble gas configuration.
What is the difference between valency and valence electron?
May 03, 2020 · Relationship between the number of valence electrons and valency: Valence electrons are the number of electrons present in the outermost shell of an atom. Valency is the combing capicity of an element to form bonds with the other elements. Click to see full answer. In this manner, what is meant by Valency of an element What is the relationship between the …
What is the relationship between group number and valence electrons?
Nov 29, 2015 · ‘Valence’, by its literary meaning in Chemistry, is related to capacity. In chemical terms, the valency of an element is the number of bonds it could form. The valence electrons would be the electrons that are available to take part in this bonding. Therefore, the main difference between valency and valence electrons is that valency is the number of bonds that …
How does the number of valence electrons affect the periodic table?
Answer : The relation between the valency of an element and the number of valence electrons in its atoms is that, the valency of an element is either equal to the number of valence electrons in its atom or equal to the number of electrons required to …
What is the valency of an atom with 7 valence electrons?
Valency is the number of bonds an element or an atom can form. Whereas valence electrons are the electrons that take part in chemical bond formation. The formation of bonds between the atoms are explained by valency. Whereas, the elemental character of an atom is explained by the valence electrons.

What is the relationship between the valency and the number of valence electrons for the elements Na to CL?
What is the relationship between number of valence electron?
Is there a relationship between the number of valence electrons of elements and their chemical and physical properties?
Is there a relationship between valency and the way elements are placed in a periodic table give one example?
What is meant by valency of an element What is the relationship between the number of valence electron and valency answer in short?
Are properties more closely related to the number of valence electrons or the number of energy levels explain your answer?
Does the number of valence electrons determine the properties of an element?
How do you find the number of valence electrons that an element has?
How are valence electrons related to each other?
Even though the two terms, Valency and Valence Electrons are very closely related to each other, there are subtle differences between the two. ‘Valence’, by its literary meaning in Chemistry, is related to capacity. In chemical terms, the valency of an element is the number of bonds it could form. The valence electrons would be the electrons that are available to take part in this bonding. Therefore, the main difference between valency and valence electrons is that valency is the number of bonds that can be formed by an atom or an element whereas valence electrons are the electrons that take part in this bond formations.
How are valence and valence related?
Even though the two terms, Valency and Valence Electrons are very closely related to each other, there are subtle differences between the two. ‘Valence’, by its literary meaning in Chemistry, is related to capacity. In chemical terms, the valency of an element is the number of bonds it could form. The valence electrons would be the electrons ...
Where are valence electrons located?
Valence electrons are the electrons that take part in bond formation. They are usually located in the outermost shell of main group elements and can even be in the closed shells of transition metals as they have multiple valencies.
What is the valency of an element?
According to the IUPAC definition, valency is ‘the maximum number of univalent atoms (originally Hydrogen or Chlorine atoms) that may combine with an atom of the element under consideration, or with a fragment, or for which an atom of this element can be substituted’.
How many valence electrons does helium have?
So, elements in group one, the alkali metals, have one valence electron, those in group two have two valence, those in group thirteen have three electrons, and so on. This is true for all elements except for helium, which is in group eight, the noble gases. It only has two valence electrons, but it still has a full outer shell like all ...
How many valence electrons are in a transition metal?
These are: Group 3: 3 valence electrons. Group 4: 2 to 4 valence electrons. Group 5: 2 to 5 valence electrons.
Is hydrogen a group element?
Hydrogen is a group element, it means it has a valency of 1. Oxygen is a group 6 element, it means it has a valency of 2…. When compounds react, they exchange their valencies..Which means that the valency of Oxygen will move to hydrogen and the valency of hydrogen will move to oxygen to form H20.
What is the valency of oxygen?
Oxygen is a group 6 element, it means it has a valency of 2…. When compounds react, they exchange their valencies..Which means that the valency of Oxygen will move to hydrogen and the valency of hydrogen will move to oxygen to form H20. H + O2-----> 2H20.
How many orbitals does phosphorus have?
In contrast Phosphorus has 9 orbitals ( one s, three p and five d ) in its valence shell and so there can be 5 unpaired electrons if one electron from the valence shell s -orbital is promoted to a d-orbital. This is how P forms 5 bonds as in PCl5, PF5 etc. Related Answer.
What is the combining power of an element?
Valency is the combining power of an element. That is the number of electron (s) will be lost (for metals) or gains (for non metals) to achieve its duplet (two electrons in outermost shell) or octet (eight electrons in outermost shell) electronic structure.
