How many exons are in the insulin receptor?
The insulin receptor has a modular structure (for review see ref. 25) encoded by a gene (located on chromosome 19) with 22 exons and 21 introns (26, Fig. 3). The short exon 11 that encodes a 12-amino acid sequence is alternatively spliced, resulting in two receptor isoforms (A and B) that differ slightly in affinity for insulin (27-29). The B isoform binds the IGFs with at least 100 times lower affinity than insulin, while the A isoform has significantly higher affinity than the B isoform for IGF-I and especially IGF-II (30) and may play a role in tumorigenesis. The IGF-I receptor binds IGF-II with a lower affinity than IGF-I and insulin with a 500-fold lower affinity. The receptors are synthesized as single chain preproreceptors that are processed by a furin-like proteolytic enzyme, glycosylated, folded and dimerized to yield the mature a 2 b 2 receptor. In cells expressing both insulin and IGF-I receptors, hybrid receptors are formed consisting of one half of each (31). Their physiological role is unknown. Comparative sequence analysis of the insulin/IGF-I receptors and the related EGF receptor (32) had led Bajaj et al. to suggest (Fig. 3) that the N-terminal half consists of two large homologous globular domains, L1 and L2, separated by a cysteine-rich region later predicted to consist of a series of disulfide-linked modules similar to those found in the tumor-necrosis factor (TNF) receptor and laminin. The C-terminal half of the receptors was predicted to consist of three fibronectin type III (FnIII) domains. The second FnIII domain contains a large insert domain (120 residues) of unknown structure containing the site of cleavage between a- and b-subunits. The disulfide bond between each a- and b- subunit involves the cysteins C647 and C860. In addition there are a-a disulfide bonds at C524 in the FnIII-1 domains and between the triplet C682-C683 and C685 in the insert domain (Fig. 3). The intracellular portion of the a-subunit contains the kinase domain flanked by two regulatory regions, a juxtamembrane region involved in docking insulin receptor substrates (IRS) 1-4 and Shc as well as in receptor internalization, and a C-terminal tail. The IGF-I receptor has a similar modular organization (33). The recent progress in the X-ray crystallographic structures of whole ectodomains or fragments of the insulin and IGF-I receptors (see below) has largely validated the structural predictions shown in Fig. 3.
What do the circles on the insulin receptors represent?
The circles marked S1 and S2 symbolize the two insulin receptor binding sites , in a symmetrical antiparallel disposition. The insulin molecule is symbolized by a black dot. a1 and a2: association rate constants for sites 1 and 2 respectively. d1 and d2: dissociation rate constants for sites 1 and 2 respectively. kcr: crosslinking constant. see text for explanations. From reference 64, used with permission.
How are insulin receptors synthesized?
The receptors are synthesized as single chain preproreceptors that are processed by a furin-like proteolytic enzyme, glycosylated, folded and dimerized to yield the mature a2b2receptor. In cells expressing both insulin and IGF-I receptors, hybrid receptors are formed consisting of one half of each (31).
How does insulin work?
The concept that insulin acts by promoting glucose transport across the membrane of target cells (rather than acting directly on enzymes of intermediary metabolism of glucose) was established in 1949 by the iconic experiment of Rachmiel Levine and colleagues (8), who showed that insulin markedly increased the volume of distribution of non-metabolisable galactose in eviscerated nephrectomized dogs from 45-47% of body weight to 75%, a figure close to that of total body water. From this finding they proposed the following working hypothesis: "Insulin acts upon the cell membrane of certain tissues (skeletal muscle, etc.) in such a manner that the transfer of hexoses (and perhaps other substances) from the extracellular fluid into the cell is facilitated. The intracellular fate of the hexoses depends upon the availability of metabolic systems for their transformation. In the case of glucose, dissimilation, glycogen storage, and transformation to fat are secondarily stimulated by the rapidity of its entry into the cell".
What is the RTK?
The RTKs are in general single transmembrane polypeptide chains that cross the cell membrane once. The intracellular part of the receptor contains a tyrosine kinase domain, inactive in the absence of ligand. In the presence of ligand bound to the extracellular domain, the RTK becomes an activated dimer, in essence an allosteric dimeric enzyme (23). There is debate as to whether the activation is due to ligand-induced dimerization or to ligand stabilization of a pre-existing monomer-dimer equilibrium (21, 23, 24). The insulin receptor subfamily (number 2 in Fig. 2), which comprizes the insulin receptor, the type 1 IGF receptor (also called IGF-I receptor) which binds insulin-like growth factors I and II, and the orphan insulin receptor-related receptor (IRRR), is an exception in the RTK superfamily in that it exists as a covalent disulfide-linked dimer (with a low basal activity kinase) in the absence of ligand. This suggests that the activation of a pre-formed dimer is a more plausible model for the superfamily. Upon ligand activation of the dimerized RTK, the kinase domains come in contact and are activated by transphosphorylation, resulting in phosphorylation of specific Tyr residues in the intracellular part of the receptor outside the kinase domain. These phosphorylated residues become binding sites for signaling partner proteins that contain SH2 (Src homology 2) domains that also become phosphorylated by the kinase or are activated by conformational changes, and start the intracellular signal transduction cascade.
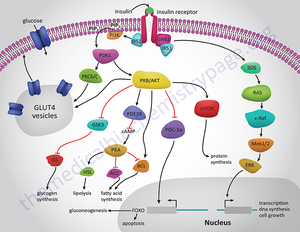
What are the pleiotropic actions of insulin?
Insulin through its receptor affects multiple physiological processes in the organism (left) by increasing (green arrows) or decreasing (red arrows) various intracellular metabolic pathways (right). Inspired by figure 2-1 of reference 1.
Where is insulin secreted?
ABSTRACT. Insulin is an anabolic peptide hormone secreted by the b cells of the pancreas acting through a receptor located in the membrane of target cells - major ones being liver (where it promotes glucose storage into glycogen and decreases glucose output), as well as skeletal muscle and fat ...
Answer
Answer - In general Insulin receptors help by providing specific shape requirements for the glucose or sugar, so that it can produce insulin and release.
New questions in Biology
50 points for anyone who answeres properly. Please answer with the options given! How does a structure of a triglyceride differ from the reaction of f …