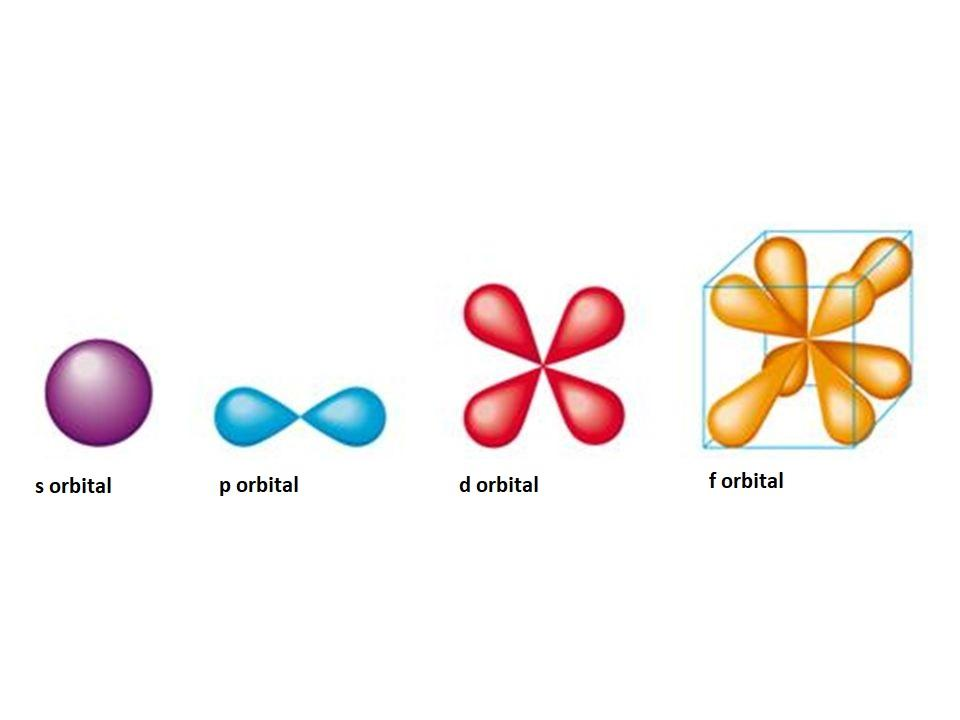
What is the shape of D and f orbitals?
What Is The Shape Of D And F Orbitals? An f orbital is an orbital for which the secondary quantum number l = 3. There are seven f orbitals, with ml = -3, -2, -1, 0, 1, 2, and 3. The f orbitals aren’t occupied in the ground state until element 58 (cerium). What is shape of F Subshell? There is no discrete shape in the f-subshell.
How many orbitals does the s subshell have?
The s subshell has only one orbital, and its general description is spherical and symmetrical. The p subshell has three orbitals, and its general shape is that of a dumbbell. The d subshell has five orbitals, and the f subshell has seven orbitals.
What is the difference between s and p subshells?
The s subshell has only one orbital, and its general description is spherical and symmetrical. The p subshell has three orbitals, and its general shape is that of a dumbbell. The d subshell has five orbitals, and the f subshell has seven orbitals. The d and f orbitals are much more complex than the s and p orbitals.
What is the shape of the s subshell of an atom?
The s subshell is spherical in shape and has one orbital. Principal shell 1n has only a single s orbital, which can hold two electrons. Principal shell 2n has one s and one p subshell, and can hold a total of eight electrons.
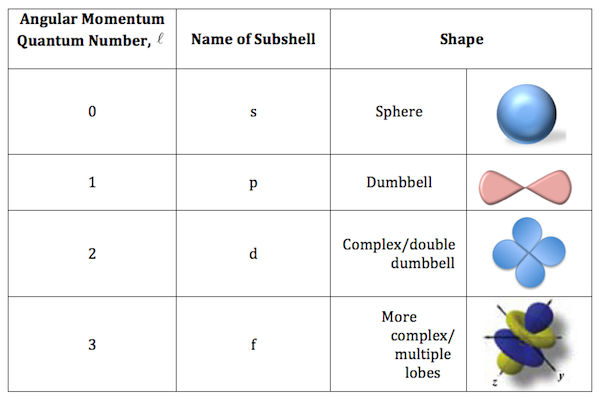
What is the shape of d and f orbitals?
The p orbital is a dumbbell shape. There are three p orbitals that differ in orientation along a three-dimensional axis. There are five d orbitals, four of which have a clover shape with different orientations, and one that is unique. There are seven f orbitals, all with different orientations.
What is the shape of d subshell?
double dumbbell-shapedHence, we can say d-orbitals have double dumbbell-shaped.
How many shapes does the F orbital have?
7f atomic orbitals general set In the general set of 7f orbitals, there are four distinct shapes, each of which possess a number of planar and conical nodes.
What is the orbital of F?
In writing the electron configuration for fluorine the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for F go in the 2s orbital. The remaining five electrons will go in the 2p orbital. Therefore the F electron configuration will be 1s22s22p5.
What is shape of p orbital?
A p orbital has the approximate shape of a pair of lobes on opposite sides of the nucleus, or a somewhat dumbbell shape.
How many orbitals are in the F subshell?
Each f subshell holds at most 14 electrons(7 orbitals)
What are the name of orbitals of F subshell?
The d subshell has 5 orbitals so can contain 10 electrons and in the f subshell there are 7 orbitals so can contain 14 electrons. So, s,p,d and f shells has 1,3,5 and 7 orbitals respectively.
How can the shapes of the f orbitals be described?
The f orbital has 15 protons to complete a fifth level of a tetrahedral structure. The f orbital is more complex, but follows the same rules based on proton alignment as the p and d orbitals. When completely full it is similar to the d orbital, but cut in half (eight lobes instead of four).
What comes after the F subshell?
After f, orbitals are simply labelled alphabetically, so the sequence is s, p, d, f, g, h, i, .... The rules for electron aufbau, i.e., how electrons are placed in orbitals, are given by the following rough scheme. 1. Electrons are always added in order of increasing energy.
Why is it called SPDF?
Spdf or SPDF may refer to: Electron configuration, for which there is an obsolete system of categorizing spectral lines as "sharp", "principal", "diffuse" and "fundamental"; also the names of the sub shells or orbitals. The blocks of the periodic table.
What is the full form of SPDF?
The names of the orbitals s, p, d, and f stand for names given to the groups of lines that are noted originally in the spectra of the alkali metals. These line groups are called the sharp, principal, diffuse, and fundamental. They are the s-orbital, p-orbital, d-orbital, and f-orbital.
What does D orbital look like?
The general shape of the d-orbitals can be described as "daisy-like" or "four leaf clover" with the exception of the the dz2 orbital which looks like the donut with a lobe above and below. All the d-orbitals contain 2 angular nodes.
What d-orbitals have the same shape?
The dxy, dyz, and dzx orbitals have the same shape i.e., cloverleaf shape but they lie in XY, YZ, and ZX planes respectively. Hence, we can say that there are five d-orbitals. These different orbitals essentially have different orientations.
How many electrons are in d subshell?
10 electronsAs d subshell has 5 orbitals and only two electrons can exist in the same orbital, therefore d subshell will have a maximum 10 electrons.
How do you describe d-orbitals?
The d orbital is a clover shape because the electron is pushed out four times during the rotation when an opposite spin proton aligns gluons with three spin-aligned protons.
What is a subshell in science?
She has taught science courses at the high school, college, and graduate levels. A subshell is a subdivision of electron shells separated by electron orbitals. Subshells are labelled s, p, d, and f in an electron configuration .
What Is a Subshell in Chemistry?
The f subshell, shown here, is partially full in atoms of Lanthanide elements. DR MARK J. WINTER, Getty Images
How many subshells are there in a shell?
Each shell consists of one or more subshells. Each subshells is composed of atomic orbitals.
Which shells have higher average energy?
Each atom has an electron shell, which is labeled K, L, M, N, O, P, Q or 1, 2, 3, 4, 5, 6, 7, moving from the shell closest to the atomic nucleus and moving outward. Electrons in outer shells have higher average energy than those in inner shells.
Which shell has a p subshell?
Each sphere is a single orbital. p subshells are made up of three dumbbell-shaped orbitals. Principal shell 2n has a p subshell, but shell 1 does not. Image Attribution: Modified by Khan Academy from OpenStax Biology ( CC BY-NC-SA 4.0)
How many orbitals does the P subshell have?
The p subshell has three dumbbell-shaped orbitals, as illustrated in the image below. Subshells d and f have more complex shapes and contain five and seven orbitals, respectively. These are not shown in the illustration below. Principal shell 3n has s, p, and d subshells and can hold 18 electrons. Principal shell 4n has s, p, d and f orbitals and can hold 32 electrons.
What is the Bohr model?
Subshells and orbitals. Recall that the Bohr model depicts an atom’s electron shell configuration. Within each electron shell are subshells, and each subshell has a specified number of orbitals containing electrons. While it is impossible to calculate exactly where an electron is located, scientists know that it is most probably located within its ...
How many electrons are in the second electron shell?
The second electron shell may contain eight electrons. This shell contains another spherical s orbital and three “dumbbell” shaped p orbitals, each of which can hold two electrons. After the 1 s orbital is filled, the second electron shell is filled, first filling its 2 s orbital and then its three p orbitals.
How many electrons can a s subshell hold?
The s subshell is spherical in shape and has one orbital. Principal shell 1n has only a single s orbital, which can hold two electrons. Principal shell 2n has one s and one p subshell, and can hold a total of eight electrons. The p subshell has three dumbbell-shaped orbitals, as illustrated in the image below.
Where are electrons located in the Bohr model?
While it is impossible to calculate exactly where an electron is located, scientists know that it is most probably located within its orbital path.
What is the difference between a shell and a subshell?
Difference Between Shell, Subshell, and Orbital: All electrons that have the same value for n (the principal quantum number) are in the same shell.Within a shell (same n), all electrons that share the same (the angular momentum quantum number, or orbital shape) are in the same subshell.
Which orbital has complex shapes with the atomic nucleus at its centre?
f orbital has complex shapes with the atomic nucleus at its centre.
What is a spherical surface within an orbital on which the probability of finding the electron is zero?
Spherical or Radial Node: A spherical surface within an orbital on which the probability of finding the electron is zero is called a spherical or radial node.
How many lobes does a P orbital have?
p orbitals have two lobes directed on opposite sides of the nucleus. P-orbitals are orientated in three different directions along X, Y and Z axis of the usual coordinate system. These orbitals are designated as P x, P y & P z orbitals. p-orbital have one nodal plane.
How many orientations does a P orbital have?
For p orbital Azimuthal quantum number l = 1 and the magnetic quantum number m = -1, 0, +1. Hence p orbitals have three orientations in space.
How many orbitals does the s subshell have?
The s subshell has only one orbital, and its general description is spherical and symmetrical. The p subshell has three orbitals, and its general shape is that of a dumbbell. The d subshell has five orbitals, and the f subshell has seven orbitals. The d and f orbitals are much more complex than the s and p orbitals.
What are the four types of subshells?
Subshells: four types: s, p, d, and f ; each has a specific number of orbitals with different shapes. Orbitals: regions within an atom that the electron will most likely occupy. Magnetic quantum number (ml): the number of orientations an orbital can have. Learning Outcomes.
What does the shape of a P orbital look like?
The shape of p orbitals, as described in the 3-dimensional plane is, in general, shaped like a dumbbell. The p orbitals have three orientations as shown here. p Orbitals.
How many subshells are there in a shell?
There are four types of subshells. Namely, s, p, d, and f. If your shell is n = 1, then the energy level is 1 and it has 1 subshell. If the n = 2, then it has 2 subshells. In this figure (see video), if n = 1, there is only one subshell, and that is s. When n = 2, there are 2 subshells, and these are s and p.
How many types of electrons are there in each shell?
Within each shell, there are subshells. The subshells have four types: s, p, d, and f, and each subshell has a specific number of orbitals with different shapes.
What are shells and subshells?
Shells and Subshells. Title. Shells and Subshells. Shells. Electrons orbit the nucleus of an atom at different ranges, called shells. Each shell has a different energy level, increasing the further it is from the nucleus. Each energy level is given a number called the principal quantum number, n. The closest shell has a value of n=1.
How many subshells are there in a shell?
Each shell must be full before the next starts to fill. This model breaks down at the n=3 shell because each shell has subshells. There are 4 subshells, s, p, d, and f. Each subshell can hold a different number of electrons. The n number determines how many of the subshells make up the shell.
How many electrons are in the first shell?
The n number determines how many of the subshells make up the shell. For example, the 1st shell is made up of 1 subshell, s. It can therefore contain only 2 electrons. The 2nd shell is made up of 2 subshells, s and p. It can therefore contain 2+6=8 electrons. A complete table for the first four shells looks like:
What does the number before each subshell do?
The number before each subshell specifies which shell it belongs to.
What is the energy level of a shell called?
Each energy level is given a number called the principal quantum number, n. The closest shell has a value of n=1. The next shell has a value of n=2, etc.
Can you write the full electron configuration in terms of subshells?
You can write the full electron configuration in terms of subshells.