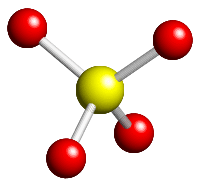
What is the Lewis structure of SO4 2?
SO4 2- Sulfate ion has two ways of drawing its lewis structure. One is to follow the octet rule and having single bond for each oxygen each perfectly satisfies the octet rule. The molecular formula for sulfate is SO42-. Four bonds, two single and two double, are shared between the sulfur and oxygen atoms.
What is the bond angle of SO4 2?
Two out of four Oxygen atoms form double bonds with Sulfur atoms. There are a total of 32 valence electrons for this ion. As all the electrons are used up in bond formation, there are no lone pairs of electrons on the sulfur atom. The bond angles are approximately 109.5 degrees.
What is the name of SO4 2?
What is the polyatomic ion so4 2 called? Description: Sulfate is a sulfur oxoanion obtained by deprotonation of both OH groups of sulfuric acid. It is a sulfur oxoanion, a sulfur oxide, an inorganic anion and a divalent inorganic anion. Likewise, what is the name of the polyatomic ion with the formula so42?
What is the electron pair geometry for SO2?
The electron geometry of SO2 is formed in the shape of a trigonal planner. The three pairs of bonding electrons arranged in the plane at the angle of 120-degree. As the one pair remained alone, two double pairs are bonded and form a bent shape. To create the Lewis structure of SO2, you need to arrange the eight valence electrons on the Sulphur.
Is SO4 2 tetrahedral in shape?
\[s{p^3}\] hybridisation- Number of sigma bonds and lone pair of electrons is 4. According to VSEPR theory the \[SO_4^{2 - }\] ion has tetrahedral molecular geometry. The \[SO_4^{2 - }\] ion has four oxygen atoms arranged tetrahedrally around the sulphur atom.
What is the shape and hybridization of SO4 2?
The shape of SO4^(2-) ion is. UPLOAD PHOTO AND GET THE ANSWER NOW! Solution : Hybridisation of `SO_4^(2-)`
` H = 1/2[6+ 0-0+2]= 4`
`therefore` The molecule has a tetrahedral shape.
What is SO4 2 Lewis structure?
5:186:25Lewis Structure of SO4(2-) (Sulfate) CORRECT - YouTubeYouTubeStart of suggested clipEnd of suggested clipWith a single bond oxygen. With a double bond.MoreWith a single bond oxygen. With a double bond.
How do you draw SO4 2?
0:004:18How to Draw the Lewis Structure for the Sulfate Ion - YouTubeYouTubeStart of suggested clipEnd of suggested clipAnd the oxygens. So there we have four bonds and we've used eight valence electrons. Just go aroundMoreAnd the oxygens. So there we have four bonds and we've used eight valence electrons. Just go around the outer atoms. And make sure they have octets. So we've used 8.
What are the bonds in so42?
There are two S=O. bonds and two S-O bonds in sulfate ion lewis structure. Sulfur atom is the center atom and four oxygen atoms are located around sulfur atom. There are no lone pairs in the last shell of sulfur atom.
What is the geometry of sio4?
tetrahedral geometrySolution : It has tetrahedral geometry.
What is the structure of so4 2 negative?
Sulphate Structure [SO42-] The sulphate ion is mainly composed of sulphur and oxygen atoms. Here, sulphur is the central atom and it is surrounded by four oxygen atoms that are located at equal distances in the plane. As for the bonding, 2 of the oxygen atoms form S=O. bonds and the other two form S-O- bonds.
Is SO42 a Lewis base?
Hence SO2 behaves as Lewis Acid as well as Lewis Base.
What type of bond is so4?
covalent bondingThe formula for this compound can be written as [Na+]2[SO42-], the sulfate anion is formed by covalent bonding between sulfur and oxygen. Since there is a bond polarity to a S-O bond these are polar covalent bonds. The attraction of the Na+ cations to SO42- is ionic. 3.
What is the structure of SO4?
SO₄²-Sulfate / Formula
Why the Valency of SO4 is 2?
The oxidation number of -2 shows that sulphate gained two electrons to complete its octet indicating its valency as 2. Thus, valency of SO4 is 2.
What is the formal charge of SO4 2?
0:002:16Calculating Formal Charges for the Sulfate Ion - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo four divided by two so six minus four that's two minus 2 that equals 0.MoreSo four divided by two so six minus four that's two minus 2 that equals 0.
What is the hybridization of sulfur in so4 2 ion is?
A The S in the SO42− ion has four electron pairs and has four bonded atoms, so the structure is tetrahedral. The sulfur must be sp3 hybridized to generate four S–O bonds.
What is the hybridization of so3 2?
The hybridization of the central atom is sp3 and the final structure of SO32- is trigonal pyramid.
What is the formal charge of so4 2?
0:002:16Calculating Formal Charges for the Sulfate Ion - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo four divided by two so six minus four that's two minus 2 that equals 0.MoreSo four divided by two so six minus four that's two minus 2 that equals 0.
What is the shape of so3 molecule?
trigonal planarIf we look at the SO3 molecular geometry it is trigonal planar with symmetric charge distribution around the central atom.
What is Sulphate?
Sulphate (SO 42-) is one of the most widely available chemical compounds accessible as naturally occurring minerals on earth. It is mostly found in the environment as a result of atmospheric and terrestrial processes. The major contributors of sulphate are sulphur released from the erosion of evaporite deposits, sulphide containing rocks and minerals and even volcanoes.
What is SO4 preparation?
SO4 Preparation. Examples. Uses of Sulphate. Effects and Hazards. In chemistry, sulphate or sulfate is taken to be an inorganic ion or polyatomic anion. Generally, sulfates are also taken to be the salts or esters of sulphuric acid. It is obtained by deprotonation of both OH groups of sulfuric acid. Additionally, it is an acid derivative ...
How many times should sulfuric acid be deprotonated?
One thing to keep in mind during the preparation of sulphate is that sulfuric acid should be deprotonated twice. If it occurs only once, then hydrogen sulfate ion is made.
What is the chemical bonding of sulfate?
Let us begin by understanding the chemical bonding and molecular structure of sulfate. The sulphate ion is mainly composed of sulphur and oxygen atoms. Here, sulphur is the central atom and it is surrounded by four oxygen atoms that are located at equal distances in the plane. As for the bonding, 2 of the oxygen atoms form S=O bonds and the other two form S-O- bonds. The oxygen atoms are responsible for the negative charge (-2) of the anion because they are in a -2 state.
What is the bonding of oxygen atoms with metal called?
This connection of a chemical compound bonding with metal is called a chelate .
What is magnesium sulfate used for?
Magnesium sulfate is used in therapeutic baths. Sulphate minerals are used in the preparation of metal salts. Copper sulfate is the most common algaecide. They are used in detergents, emulsifiers, and foaming agents. Gypsum is a natural form of hydrated calcium sulfate used in the making of plasters.
What angle is sulfate placed at?
Structure of Sulphate. The atoms are placed at a 109.5° angle. In order to determine the structure students can learn to draw the Lewis structure of sulfate and also know about the formal charges as well as the total number of valence electrons needed for sulfate.
How many valence electrons does each atom need to have to be stable?
Now that you have the arrangement of the atoms in the molecule, we can look at the bonding of atoms. As mentioned above, each atom will try to have eight valence electrons in its outer shell to attain a stable structure.
How many pairs of electrons are there in sulfur?
As all the electrons are used up in bond formation, there are no lone pairs of electrons on the sulfur atom. The bond angles are approximately 109.5 degrees. It has a tetrahedral molecular shape and -2 charge as it accepts two additional electrons to attain a stable structure. The -2 charges are due to the two Oxygen atoms forming ...
Why do sulfur atoms have 2 charges?
The -2 charges are due to the two Oxygen atoms forming a single bond with the Sulfur atom.
How to determine the geometry of a molecule?
We can determine the molecular geometry of any given molecule using the VSEPR theory model and the AXN notation method. For example, for the Sulphate ion, the AXN notation would be AX4, as it forms bonds with four oxygen atoms. And as a result of this, it has a tetrahedral molecular geometry.
How many electrons does sulfur share with oxygen?
Start putting these electrons around the Oxygen atom, as Sulphur has shared four electrons with the Oxygen atoms. Now doing this, you might wonder that this is the correct way to present the Lewis Structure, but it is suggested to calculate the formal charges to do so.
What is SO42- a sulfate?
SO42- is a chemical name of the sulfate ion. It comprises one Sulphur atom, four Oxygen atoms and a charge of -2. It is a polyatomic anion and is used widely to synthesize other sulfates such as Zinc Sulfates, Magnesium sulfates, Iron sulfates and much more. It is also a sulfate salt for sulphuric acids. As this molecule has many applications in various industries today, it is vital to know its Lewis Structure, Molecular Geometry, and more.
What is the charge of sulfur if there are 4 bonds?
Using this formula, you will find out that when there are four single bonds in this molecule, each Oxygen atom has a charge of -1, and the Sulphur atom has the charge of -2. So if you add up the charges, it is 4 (-1)+ (-2) = -6, which is technically wrong as we have a charge of -2 on this ion.
How many valence electrons are in SO42?
Step 1: Count the total number of valence electrons present in the molecule/ion. For sulfate ions, we have one molecule of sulfur and four molecules of oxygen. Sulfur and oxygen both belong to the same group in the periodic table ( the chalcogen family) and have six valence electrons each. total valence electrons in SO42- = 6*1 + 6*4 +2 = 32.
Why did we have to add two electrons to sulfate?
Note: We had to add two electrons due to the negative 2- charge on sulfate ion.
Why is sulfate ion important?
Sulfate ion found in salts naturally and industrially prepared is an important chemical component that we need to learn. We have and will come across a lot of compounds in the future that will bear sulfate ions. So it is necessary to have some knowledge about it to comprehend the possible reactions and physical and chemical properties of the respective compounds. Chemical bonding helps us have a solid and smooth idea about the nitty-gritty of atomic components.
What is hybridization in biology?
Hybridization is an essential concept of chemical bonding, something that will be handy anytime anywhere when we need to talk about a molecule or learn about its properties.
What is the charge of SO42?
Sulfate ion (SO42-) is one of the most common ions that people in chemistry need to deal with. This is a polyatomic anion having a negative charge of -2.
What is the formal charge of sulfur?
Now, if we check the formal charges, we will find out that the formal charge for sulfur is zero, that of the doubly bonded oxygen atoms is zero, and that of the singly bonded oxygen atom s is -1.
How do we measure polarity?
We measure polarity via a term called a dipole moment.
