
What is the shape of orbitals and sublevels?
Shapes of Orbitals and Sublevels. Orbitals are the region of the atom where there is a 90% probability of finding and electron. Orbitals hold 2 electrons. The s-sublevel is made up of a singular orbital holding a maximum of 2 electrons. It is a spherical shape.
How many orbitals are there in the D sublevel?
The d sublevel has 5 orbitals, so max. 10 electrons can be present. And the 4 sub-levels have seven orbitals, and they can hold max 14 electrons. Why is the s orbital spherical? All s orbitals are shaped spherically and have spherical symmetry.
What is the shape of the s-sublevel?
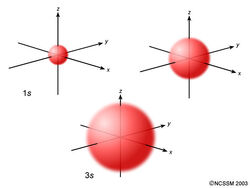
The s-sublevel is made up of a singular orbital holding a maximum of 2 electrons. It is a spherical shape. The 1s is the closest to the nucleus and is smaller that the 2s, which is smaller than the 3s and so on.
What is the shape of d orbitals?
The Shape of d Orbitals 1 The magnetic orbital quantum number for d orbitals is given as (-2,-1,0, 1,2). ... 2 These orbitals are designated as d xy, d yz, d xz, d x2–y 2 and d z2. 3 Out of these five d orbitals, shapes of the first four d-orbitals are similar to each other, which is different from the d z2 orbital whereas the energy of all ...
What is the shape of the f sublevel?
F-subshell: This is the fourth subshell in the segment where there is maximum space of 7 orbitals and 14 electrons. There is no discrete shape in the f-subshell. This is because of the complexity of its nature. Therefore the answer would be option D, No definite shape.
What is the shape of the D level?
clover shapeThe d orbital is a clover shape because the electron is pushed out four times during the rotation when an opposite spin proton aligns gluons with three spin-aligned protons.
What is the shape of sublevel s?
The s-sublevel is made up of a singular orbital holding a maximum of 2 electrons. It is a spherical shape. The 1s is the closest to the nucleus and is smaller that the 2s, which is smaller than the 3s and so on. The p-sublevel is made up of a 3 identical dumbbell like orbitals.
How many sublevels does D have?
Number of electrons per sublevelEnergy LevelSublevelsNumber of Orbitals4s1p3d5f76 more rows
How many shapes represent the d orbital?
five d-orbitalsThe Shape of d Orbitals Hence, we can say that there are five d-orbitals. These orbitals are designated as dxy, dyz, dxz, dx2–y 2 and dz2.
What is the structure of d orbital?
d-orbital has 5 orientations : dxy,dyz,dzx,dx−y and dz.
What is the shape of d and f orbitals?
The p orbital is a dumbbell shape. There are three p orbitals that differ in orientation along a three-dimensional axis. There are five d orbitals, four of which have a clover shape with different orientations, and one that is unique. There are seven f orbitals, all with different orientations.
What is shape of p orbital?
A p orbital has the approximate shape of a pair of lobes on opposite sides of the nucleus, or a somewhat dumbbell shape.
What are the shapes of the 4 sublevels?
Sublevels. Each of the four sublevels have a specific shape or area where electrons can be found. As shown in Figure 3.6, the s sublevel has a spherical shape, the p sublevel has a dumbbell shape, and the d sublevel has a four leaf clover shape.
How many orbitals are in D sublevel?
5 orbitalsThe d sublevel has 5 orbitals, so can contain 10 electrons max.
What is meant by d-orbital?
Beginning in the third energy level, aset of five degenerate orbitals per energy level, higher in energy than s and p orbitals of the same energy level. Search the Dictionary for More Terms.
What is the name of d-orbital?
The five d - orbitals are designated as dxy, dyz, dxz, dx^2 - y^2 and dz^2 .
What is a 2D shape?
2D shapes are shapes with two dimensions, such as width and height. An example of a 2D shape is a rectangle or a circle. 2D shapes are flat and cannot be physically held, because they have no depth; a 2D shape is completely flat.
Which is not a 2D shape?
A sphere is not a 2-dimensional shape. These are also flat and can be drawn or structured on any flat surface. A triangle, rectangle, circle, square, etc.
What is 2D example?
Examples of 2D Geometric Shapes A circle, triangle, square, rectangle, and pentagon are all examples of two-dimensional shapes.
How do you sort shapes?
0:031:04Sorting by Shape - YouTubeYouTubeStart of suggested clipEnd of suggested clipWhen a child has learned some basic shapes. You can help deepen their understanding through sorting.MoreWhen a child has learned some basic shapes. You can help deepen their understanding through sorting. You can give the child some shapes cut from paper making sure there are a few of each shape.
What is the p sublevel?
The p-sublevel is made up of a 3 identical dumbbell like orbitals. Each one is situated on its own axis. They are at 90 o angles from one and other.
How many electrons are in the S-sublevel?
The s-sublevel is made up of a singular orbital holding a maximum of 2 electrons. It is a spherical shape.
