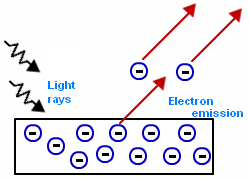
Thus, photoelectric effect is important.
- It helps in explaining the particle nature of light.
- It is used in the photocells, such as calculators, alarms, solar cells, etc.
- The photoelectric effect is widely used in electronic devices which are specialized in the emission of electrons in a precisely timely manner.
- This effect is used to detect the presence of light.
Which theory best explains the photoelectric effect?
The photoelectric effect is an instantaneous process. As soon as light hits the surface, the electrons of the metal come out. Planck’s Theory and the Photoelectric Effect. Planck's theory was expanded by Einstein in 1905 to explain the photoelectric effect, which is the release of electrons by metal when exposed to light or high photons.
How did the photoelectric effect change the world?
How did the photoelectric effect change the world? Light with energy above a certain point can be used to knock electrons loose, freeing them from a solid metal surface, according to Scientific American. Applications of the photoelectric effect brought us “electric eye” door openers, light meters used in photography, solar panels and ...
Does the photoelectric effect require a battery or a circuit?
Re: Does the photoelectric effect require a battery or a circuit? The effect itself does not require a biasing source (battery) but to reliably observe the effect does require the biasing voltage.
How can the photoelectric effect can be explained?
The photoelectric effect can be explained by ( ... According to Einstein's photoelectric equation the plot of the kinetic energy of the emitted photo-electrons from a metal vs the frequency of the incident radiation gives a straight line whose slope ( ...

What is the significance of photoelectric?
The photoelectric effect is the process that involves the ejection or release of electrons from the surface of materials (generally a metal) when light falls on them. The photoelectric effect is an important concept that enables us to clearly understand the quantum nature of light and electrons.
What is the importance of photoelectric effect in our daily life?
The phenomenon of photoelectric effect is used to generate electricity with the help of solar panels. The solar panel contains metal which helps to generate electricity by releasing energy when the light hits the metal.
What is photoelectric effect and its applications?
photoelectric effect, phenomenon in which electrically charged particles are released from or within a material when it absorbs electromagnetic radiation. The effect is often defined as the ejection of electrons from a metal plate when light falls on it.
What is the conclusion of photoelectric effect?
Hence, the conclusion of the photoelectric effect is that the energy in light comes in small packets.
What was the most significant result of the photoelectric effect?
Study of the photoelectric effect led to important steps in understanding the quantum nature of light and electrons and influenced the formation of the concept of wave–particle duality.
How is the photoelectric effect used in the medical field?
The photoelectric effect is the most important effect in medical radiography. E.g. it is photoelectric absorption that is responsible for most of the absorption in a mammogram which creates the contrast in the image. See also Photon, Electron.
How is photoelectric effect used in astronomy?
These devices utilise the photoelectric effect to convert incident photons into a charge that can then be measured and recorded. CCDs have revolutionised astronomy in the last two decades, virtually replacing photography in professional astronomy.
Is the photoelectric effect used in solar panels?
Photovoltaic solar energy is generated by converting sunlight into energy, a type of clean, renewable, and inexhaustible energy that can be produced in installations ranging from small panels on the top of houses to large photovoltaic plants. This is achieved using a technology based on the photoelectric effect.
What is the photoelectric effect?
photoelectric effect, phenomenon in which electrically charged particles are released from or within a material when it absorbs electromagnetic radiation. The effect is often defined as the ejection of electrons from a metal plate when light falls on it. In a broader definition, the radiant energy may be infrared, visible, or ultraviolet light, ...
What is the energy band of an atom?
The highest energy configuration (or energy band) that is normally occupied by electrons for a given material is known as the valence band , and the degree to which it is filled largely determines the material’s electrical conductivity. In a typical conductor (metal), the valence band is about half filled with electrons, which readily move from atom to atom, carrying a current. In a good insulator, such as glass or rubber, the valence band is filled, and these valence electrons have very little mobility. Like insulators, semiconductors generally have their valence bands filled, but, unlike insulators, very little energy is required to excite an electron from the valence band to the next allowed energy band—known as the conduction band, because any electron excited to this higher energy level is relatively free. For example, the “bandgap” for silicon is 1.12 eV ( electron volts ), and that of gallium arsenide is 1.42 eV. This is in the range of energy carried by photons of infrared and visible light, which can therefore raise electrons in semiconductors to the conduction band. (For comparison, an ordinary flashlight battery imparts 1.5 eV to each electron that passes through it. Much more energetic radiation is required to overcome the bandgap in insulators.) Depending on how the semiconducting material is configured, this radiation may enhance its electrical conductivity by adding to an electric current already induced by an applied voltage ( see photoconductivity ), or it may generate a voltage independently of any external voltage sources ( see photovoltaic effect ).
How does photoconductivity occur?
Photoconductivity arises from the electrons freed by the light and from a flow of positive charge as well. Electrons raised to the conduction band correspond to missing negative charges in the valence band, called “holes.”. Both electrons and holes increase current flow when the semiconductor is illuminated.
What is the energy of a photon?
Consideration of these unexpected behaviours led Albert Einstein to formulate in 1905 a new corpuscular theory of light in which each particle of light, or photon, contains a fixed amount of energy, or quantum, that depends on the light’s frequency. In particular, a photon carries an energy E equal to hf, where f is the frequency of the light and h is the universal constant that the German physicist Max Planck derived in 1900 to explain the wavelength distribution of blackbody radiation—that is, the electromagnetic radiation emitted from a hot body. The relationship may also be written in the equivalent form E = hc /λ, where c is the speed of light and λ is its wavelength, showing that the energy of a photon is inversely proportional to its wavelength.
How does illumination affect photoelectric energy?
Other photoelectric effects are caused by radiation at higher frequencies, such as X-rays and gamma rays.
How is voltage generated in photovoltaics?
In the photovoltaic effect, a voltage is generated when the electrons freed by the incident light are separated from the holes that are generated , producing a difference in electrical potential. This is typically done by using a p - n junction rather than a pure semiconductor.
What happens when an inner electron is ejected?
When such an inner electron is ejected, a higher-energy outer electron quickly drops down to fill the vacancy. The excess energy results in the emission of one or more additional electrons from the atom, which is called the Auger effect.
Significance of the photoelectric effect
Could someone explain the significance of the photoelectric effect, and how to solve problems using E (photon) - E (energy to remove e-) = E (excess) = EK (e-) = 1⁄2 mv2?
Re: Significance of the photoelectric effect
The photoelectric effect is significant because it demonstrates that light has particle-like qualities. It established that we can consider light as photons (packets) of energy where one photon interacts w/ one electron and each photon must have sufficient energy to remove each electron.
Re: Significance of the photoelectric effect
I believe the photoelectric effect's significance stems from showing how light rejects the traditional wave model, and how light is instead perceived as photons/quanta of energy . According to the wave model, if the amplitude increases then the intensity of the light wave should increase and eject more electrons.
Re: Significance of the photoelectric effect
The photoelectric effect is significant because it showed that light has both wave-like properties and particle-like properties. The finding was that increasing the intensity of the light would not result in electrons being emitted from the metal surface.
Lenard's Experimental Results (Intensity Dependence)
In 1902, Hertz's student, Philipp Lenard, studied how the energy of the emitted photoelectrons varied with the intensity of the light. He used a carbon arc light and could increase the intensity a thousand-fold.
Millikan's Experimental Results (Wavelength Dependence)
The American experimental physicist Robert Millikan followed up on Lenard's experiments and using a powerful arc lamp, he was able to generate sufficient light intensity to separate out the colors and check the photoelectric effect using light of different colors.
Einstein's Quantum Picture
In 1905 Einstein gave a very simple interpretation of Lenard's results and borrowed Planck's hypothesis about the quantized energy from his blackbody research and assumed that the incoming radiation should be thought of as quanta of energy h ν, with ν the frequency. In photoemission, one such quantum is absorbed by one electron.
The Workfunction (Φ)
The workfunction is an intrinsic property of the metal. While the workfunctions and ionization energies appear as similar concepts, they are independent. The workfunction of a metal is the minimum amount of energy ( E) necessary to remove an electron from the surface of the bulk ( solid) metal (sometimes referred to as binding energy ).
Summary
Although Hertz discovered the photoelectron in 1887, it was not until 1905 that a theory was proposed that explained the effect completely.
Conceptual Questions
Is visible light the only type of electromagnetic radiation that can cause the photoelectric effect?
Contributors and Attributions
Paul Flowers (University of North Carolina - Pembroke), Klaus Theopold (University of Delaware) and Richard Langley (Stephen F. Austin State University) with contributing authors. Textbook content produced by OpenStax College is licensed under a Creative Commons Attribution License 4.0 license.