What does C8H18 stand for in molecular compounds?
What is the empirical formula for octane c8h18? CHEBI:17590 - octane Octane is a hydrocarbon and an alkane with the chemical formula C8H18 , and the condensed structural formula CH3(CH2)6CH3. Octane has many structural isomers that differ by the amount and location of branching in the carbon chain.
What is the balanced chemical equation for C3H8?
The balanced chemical equation for C3H8 +O2 = CO2 + H2O is C3H8 +5O2 = 3CO2 +4H2O. This is the chemical reaction in which C3H8 or propane burns in air or oxygen to produce carbon dioxide and water. This chemical reaction is a complete combustion of a hydrocarbon in the presence of excess oxygen.
Which reaction represents the enthalpy of formation?
standard enthalpy of reaction: The enthalpy change that occurs in a system when one mole of matter is transformed by a chemical reaction under standard conditions. The standard enthalpy of reaction, [latex]Delta H^ominus _{rxn}latex], is the change in enthalpy for a given reaction calculated from the standard enthalpies of formation for all reactants and products.
What is standard state of oxygen in enthalpy of formation?
The standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as ozone (O3), atomic oxygen (O), and molecular oxygen (O2), O2 is the most stable form at 1 atm pressure and 25°C.

What is the standard enthalpy of formation of C8H18 G?
Selected ATcT enthalpy of formation based on version 1.118 of the Thermochemical Network Species NameFormulaΔfH°(298.15 K)OctaneC8H18 (l)-249.73
How do you find the standard enthalpy of formation?
This equation essentially states that the standard enthalpy change of formation is equal to the sum of the standard enthalpies of formation of the products minus the sum of the standard enthalpies of formation of the reactants. and the standard enthalpy of formation values: ΔH fo[A] = 433 KJ/mol. ΔH fo[B] = -256 KJ/mol.
What is the standard enthalpy of combustion of octane?
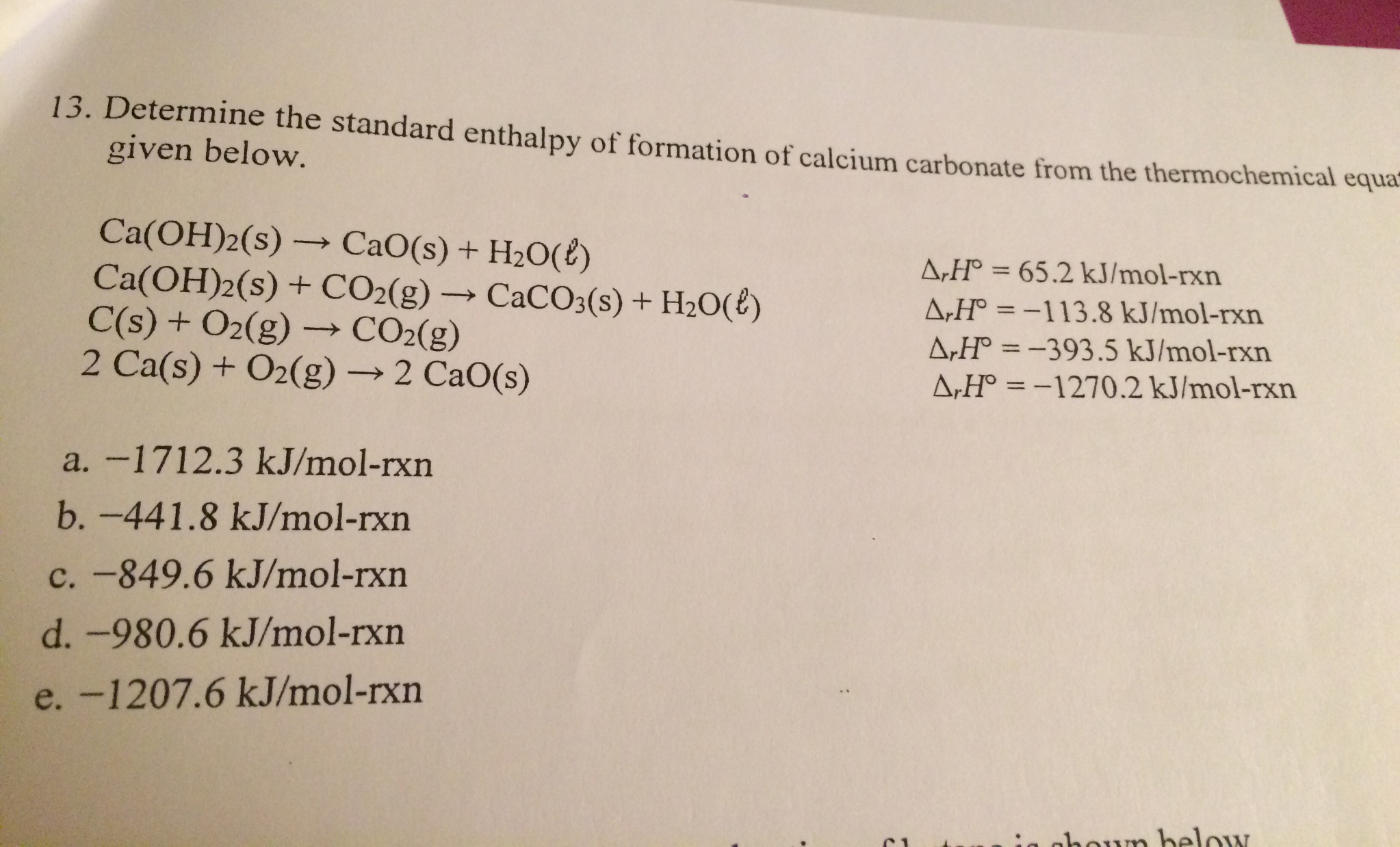
5470 kJ/molThe standard heat of combustion of octane is 5470 kJ/mol. This is the heat released when octane is fully combusted under standard conditions. As such, the standard enthalpy change for the complete combustion of octane is -5470 kJ/mol.
What is the standard enthalpy of formation for C2H6?
Enthalpy of formation of C2H6=−83kJ/mol.
Why the standard enthalpy of formation is zero?
And since there's no change, there's no change in enthalpy. Therefore, the standard enthalpy of formation is equal to zero. And this is true for the most stable form of any element. The standard enthalpy of formation of the most stable form of any element is zero since you'd be making it from itself.
What do you mean by standard enthalpy of formation?
Standard enthalpy of formation is defined as the enthalpy change when one mole of a compound is formed from its elements in their most stable state of aggregation (stable state of aggregation at temperature: 298.15 K, pressure: 1 atm).
How do you calculate the enthalpy of formation of octane?
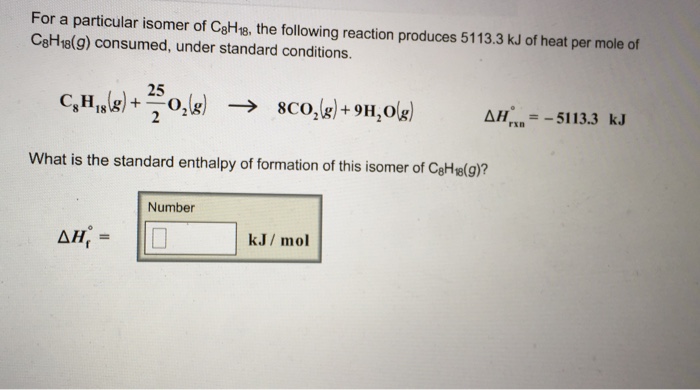
C8H18(g)+252O2(g)→8CO2(g)+9H2O(l)ΔrH∘=8×(−394)+9×(−286)−(−250)=−5476 kJ/mol.
What is enthalpy of combustion give one example?
Standard enthalpy of combustion (ΔH∘C) is the enthalpy change when 1 mole of a substance burns (combines vigorously with oxygen) under standard state conditions; it is sometimes called “heat of combustion.” For example, the enthalpy of combustion of ethanol, −1366.8 kJ/mol, is the amount of heat produced when one mole ...
Is the formation of octane exothermic?
The fuel can be almost anything including methane (CH4), propane (C3H8), butane (C4H10), octane (C8H18) or sugar (C6H12O6). The combustion of methane or octane is exothermic; it releases energy.
What is the enthalpy of formation for H2?
For H2 enthalpy of formation is zero, because it already is the most elementary form.
What is the entropy of C2H6?
P(s)3. 41.1. C2H6(g) 229.2.
What is the enthalpy of formation of CH4?
The standard enthalpy of formation of CH4(g), CO2(g) and H2O(g) are -76.2, -394.8 and -241.6 kJ/mol respectively.
What is the formula to calculate enthalpy?
If you want to calculate the enthalpy change from the enthalpy formula:Begin with determining your substance's change in volume. ... Find the change in the internal energy of the substance. ... Measure the pressure of the surroundings. ... Input all of these values to the equation ΔH = ΔQ + p * ΔV to obtain the change in enthalpy:More items...•
How do you find the enthalpy of formation of bond energies?
0:197:48Using Average Bond Enthalpies - Chemistry Tutorial - YouTubeYouTubeStart of suggested clipEnd of suggested clipWe need to take the sum of the bond enthalpies of the bonds broken and subtract the sum of the bondMoreWe need to take the sum of the bond enthalpies of the bonds broken and subtract the sum of the bond enthalpies of the bonds formed.
How do you calculate the enthalpy of a reaction?
Use the formula ∆H = m x s x ∆T to solve. Once you have m, the mass of your reactants, s, the specific heat of your product, and ∆T, the temperature change from your reaction, you are prepared to find the enthalpy of reaction. Simply plug your values into the formula ∆H = m x s x ∆T and multiply to solve.
What formula is Q MC ∆ T?
The amount of heat gained or lost by a sample (q) can be calculated using the equation q = mcΔT, where m is the mass of the sample, c is the specific heat, and ΔT is the temperature change.
What is the standard enthalpy of formation?
The standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy during the formation of 1 mole of the substance from its constituent elements, with all substances in their standard states. The standard pressure value p⦵ = 10 5 Pa (= 100 kPa = 1 bar) is recommended by IUPAC, ...
How is the enthalpy of formation measured?
The standard enthalpy of formation is measured in units of energy per amount of substance, usually stated in kilojoule per mole (kJ mol −1 ), but also in kilocalorie per mole, joule per mole or kilocalorie per gram (any combination of these units conforming to the energy per mass or amount guideline).
How to calculate the standard enthalpy of a reaction?
The standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of reactants and products using Hess's law. A given reaction is considered as the decomposition of all reactants into elements in their standard states, followed by the formation of all products. The heat of reaction is then minus the sum of the standard enthalpies of formation of the reactants (each being multiplied by its respective stoichiometric coefficient, ν) plus the sum of the standard enthalpies of formation of the products (each also multiplied by its respective stoichiometric coefficient), as shown in the equation below:
What is the enthalpy of all elements in their standard state?
All elements in their standard states ( oxygen gas, solid carbon in the form of graphite, etc.) have a standard enthalpy of formation of zero, as there is no change involved in their formation.
What is the formula for combustion of methane?
For example, for the combustion of methane, CH 4 + 2 O 2 → CO 2 + 2 H 2 O:
What is the standard state of a substance?
For a pure substance or a solvent in a condensed state (a liquid or a solid): the standard state is the pure liquid or solid under a pressure of 1 bar. For an element: the form in which the element is most stable under 1 bar of pressure. One exception is phosphorus, for which the most stable form at 1 bar is black phosphorus, ...
Do allotropes have enthalpy?
Allotropes of an element other than the standard state generally have non-zero standard enthalpies of formation.