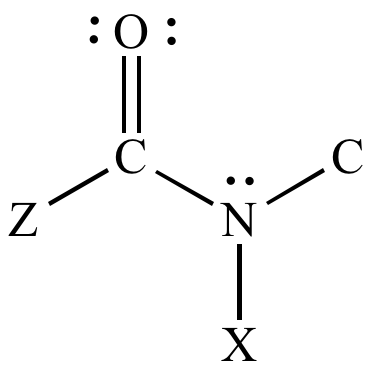
What is amide formula?
Amide groups have the general chemical formula CO-NH.
What is the shape of amide?
TRIGONAL PLANARThe center of the amide functional group is the carbon double bond oxygen and the nitrogen. With three atoms attached to this carbon, the molecular geometry is TRIGONAL PLANAR. This portion of the molecule is flat, with bond angles of 120 degrees.
What is an amide?
An amide is a functional group containing a carbonyl group linked to a nitrogen atom or any compound containing the amide functional group. Amides are derived from carboxylic acid and an amine. Amide is also the name for the inorganic anion NH2. It is the conjugate base of ammonia (NH3).
How do you draw an amide?
8:2710:14Examples for naming and drawing amides - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo there's the general structure. And always need to fill in the remaining bonds. And we'll get toMoreSo there's the general structure. And always need to fill in the remaining bonds. And we'll get to that when we get back to the name. The next part is the parent. Name.
What is amine structure?
Amines resemble ammonia structurally where nitrogen can bond up to 3 hydrogen atoms. It is also characterized by various properties that are based on carbon connectivity. Compounds of nitrogen connected to a carbonyl group are called as amides, they have a structure R–CO–NR′R″ and vary in properties with amines.
Are amides planar?
Amides are neutral compounds -- in contrast to their seemingly close relatives, the amines, which are basic. The amide linkage is planar -- even though we normally show the C-N connected by a single bond, which should provide free rotation.
How do you name amide?
Primary amides are named by changing the name of the acid by dropping the -oic acid or -ic acid endings and adding -amide. The carbonyl carbon is given the #1 location number.
Is amide a base?
Basicity. Compared to amines, amides are very weak bases. While the conjugate acid of an amine has a pKa of about 9.5, the conjugate acid of an amide has a pKa around −0.5. Therefore, amides don't have as clearly noticeable acid–base properties in water.
Is an amide an amine?
No, amine and amide are not the same. Amine is an ammonia derivative in which one or more hydrogen atoms are replaced by an alkyl or aryl group, while amide is an amine derivative of carboxylic acid. A sigma bond joins a carbonyl carbon atom to a nitrogen atom bonded by hydrogen atoms or carbon atoms.
What is the functional group of amide?
An amide is a functional group that consists of a carbonyl group and nitrogen atom and can be derived from the various functional group known as carboxylic acid.
Why is amide trigonal planar?
In the unique third contributing structure of an amide, the lone pair on nitrogen donates electron density to create a pi bond between carbon and nitrogen. Inclusion of the third resonance contributing structure explains why the amide nitrogen is sp2 hybridized and therefore trigonal planar.
How do you read amide?
As with amines, the nomenclature used for an amide depends on the number of carbons attached to the nitrogen. A primary (1°) amide has nitrogen attached to a single carbon; a secondary (2°) amide has the nitrogen attached to two carbons; a tertiary (3°) amide has the nitrogen attached to three carbons.
Why is amide trigonal planar?
In the unique third contributing structure of an amide, the lone pair on nitrogen donates electron density to create a pi bond between carbon and nitrogen. Inclusion of the third resonance contributing structure explains why the amide nitrogen is sp2 hybridized and therefore trigonal planar.
What are the properties of amides?
Like the esters, solutions of amides in water usually are neutral—neither acidic nor basic. The amides generally have high boiling points and melting points. These characteristics and their solubility in water result from the polar nature of the amide group and hydrogen bonding (Figure 15.14.
What is difference between amine and amide?
Amine is an ammonia derivative in which one or more hydrogen atoms are replaced by an alkyl or aryl group, while Amide is an amine derivative of carboxylic acid. A sigma bond joins a carbonyl carbon atom to a nitrogen atom bonded by hydrogen atoms or carbon atoms.
How do you name amide?
Primary amides are named by changing the name of the acid by dropping the -oic acid or -ic acid endings and adding -amide. The carbonyl carbon is given the #1 location number.
What is an amide vs Amine?
An amide differs from an amine in both its structure and properties. An amide consists of an acyl group attached to the NH2 group, while an amine c...
What are the characteristics of amides?
Amides consist of a carbonyl group attached to the NH2 group. The amide group is polar due to the presence of the NH2 group. The NH2 group can form...
What is an amide group?
An amide group is an organic compound which consists of a carbonyl group attached to an amine group. The amide functional group is given as -CONH2.
What is an amide?
An amide is a functional group that contains a nitrogen atom and carbonyl group. As we will see, they can be derived from a different functional group called a carboxylic acid. {"error":true,"iframe":true}. You must c C reate an account to continue watching. Register to view this lesson.
How to make an amide?
We will briefly summarize the process it takes to make an amide. Carboxylic acid gets converted into an ammonium salt with the help of an amine. By heating this intermediate (the ammonium salt), it forms the desired product, an amide. Keep in mind that to be derived (i.e. made) from a carboxylic acid, the creation of this intermediate is very important. As we'll see, it is this step that leads us to the formation of our friend, amide.
What is the difference between amide and carbonyl?
Differences in these types depend on the location of the nitrogen atom attached to the carbon atom in a molecular chain. The amide linkage consists of a single bond between the carbon atom in the carbonyl group and nitrogen atom. An amide can be synthesized from different starting compounds such as acid chlorides and nitriles.
What is the amide group in Tylenol?
Now that we know what an amide looks like, we can identify the amide group in the structure of Tylenol. The chemical name of Tylenol is paracetamol. Can you locate the amide group? It is the region of the compound where the nitrogen atom is attached to a carbonyl group. To test yourself, what type of amide is this? If you guessed that it is specifically a secondary amide, you're on a roll!
How to name secondary amide?
A secondary amide is named by adding an upper case N to let you know a nitrogen atom is attached to an alkyl group. An alkyl group is a type of hydrocarbon chain containing carbon and hydrogen atoms. The naming of a tertiary amide (in case you see one of them) follows the same guidelines.
What is the intermediate for the formation of amide?
Specifically, the formation of amide from a carboxylic acid must involve the creation of an intermediate called an ammonium salt. Heating of this intermediate leads to the formation of an amide. To unlock this lesson you must be a Study.com Member. Create your account.
How many types of amides are there?
Three Types of Amides. When you run into an amide, whether it's the structure or name, here are some points to keep in mind regarding nomenclature. Amides can be classified as three different types, according to naming: primary, secondary, or tertiary.
What are amides made of?
Amides are composed of one nitrogen, one carbonyl group carbon, one oxygen atom, and a variable number of hydrogen and carbon atoms. The hybridisation of carbonyl carbon is sp2 and the hybridization of nitrogen in the amide group is sp2. The Sp2 hybridization is due to the resonance. The sp2 hybridisation attains the planar structure of the nitrogen bond. In the amide structure, one lone pair is present on the nitrogen group. In an amide structure, the oxygen atom is attached to the carbon by two double bonds. The lone pair and carbonyl group are arranged in an alternate manner. This contributes to the resonance in the molecule. The presence of a double bond restricts the rotation about the nitrogen linkage.
What is the amide formula?
The amide group contains a carboxyl group and one nitrogen group. The amide formula is R-C (O)-N-H2, R-C (O)-N-R2, and R-C (O)-N-HR. Some examples of amides are acetamide, benzamide, and diethylformmide.
Why are amides amphoteric?
Due to the partial double bond, amides exhibit planner restriction. Amides are amphoteric in nature, acting as both acid and base. The presence of a high electronegative element (oxygen) in the amide group, amides participate in the hydrogen bonding. Amides undergo several chemical reactions.
Why do amides have hydrogen bonds?
Due to the presence of a highly electronegative element in the amide group, they exhibit hydrogen bonding.
What chemical reaction is used to make amides?
These chemical reactions include Hofmann rearrangement reaction, amide reduction reaction, Vilsmeier- Haack reaction. Hydrolysis Reaction- - When amides heated with water, acids, or alkalies, it gives a hydrolysis reaction and forms carboxylic acids and free ammonia as a product.
How many double bonds are there in an amide?
In an amide structure, the oxygen atom is attached to the carbon by two double bonds. The lone pair and carbonyl group are arranged in an alternate manner. This contributes to the resonance in the molecule. The presence of a double bond restricts the rotation about the nitrogen linkage.
What is the reaction of amide and hydrochloric acid?
When amide is heated with hydrochloric acid and sodium nitrite, it gives effervescence due to the evolution of nitrogen.
What is an Amide?
Amides are compounds that consist of a carbonyl functional group which is connected to both an amine group and a hydrocarbon group (or hydrogen atom). A carbonyl functional group consists of a carbon atom which is double-bonded with an oxygen atom.
What is the simplest amide?
Acetamide is the simplest amide. It consists of a methyl group connected to the carbonyl carbon of the amide.
How does an amide differ from an amine?
An amide differs from an amine in both its structure and properties. An amide consists of an acyl group attached to the NH2 group, while an amine consists of an alkyl group attached to NH2.
What is secondary amide?
A secondary amide is one in which the nitrogen atom of an amide is connected to a hydrocarbon substituent.
What is the reaction of an amine with an acyl group (a carboxylic acid derivative)?
The reaction of an amine with an acyl group (a carboxylic acid derivative) generates an amide.
How are primary amines formed?
Primary amines are formed by replacing a hydrogen atom of ammonia with a hydrocarbon substituent.
How is an amine formed?
An amine is a functional group formed by the replacement of the hydrogen atoms of the ammonia molecule with a hydrocarbon substituent.
What are amides in functional groups?
As you know from the functional groups, amides are the “combination” of the carbonyl and amines: Depending on the number of carbons connected to the nitrogen, we have primary, secondary and tertiary amides. Notice that the carbonyl carbon is also counted:
What are the applications of amides?
The applications of amides are not limited to biological systems only. In addition to having the exceptional properties as a covalent bond, the peptide bond displays strong intermolecular interactions such as the hydrogen bonding of the carbonyl groups. This, together with the aromatic π-π stacking interactions, are indispensable for making Kevlar (used for production of bullet proof materials), Nylon, and other synthetic polymers:
Why is the amide bond stable?
Stability of the Amide Bond. The amide bond (amide linkage, peptide bond) is the linking unit in proteins which are polymers of amino acids. And, by definition, this bond must be stable in aqueous cellular environment and in other biological systems: There are two main reasons for this. First is the fact that they have the poorest leaving group ...
Why do amines have poor leaving groups?
First is the fact that they have the poorest leaving group (–NH2, RNH–, R2N–) because the p K a of amines ranges from 32-38 and their conjugate bases are very strong bases and, therefore, poor leaving groups:
Which is the least reactive carboxylic acid derivative in nucleophilic acyl substitution reactions?
This, together with the aromatic π-π stacking interactions, are indispensable for making Kevlar (used for production of bullet proof materials), Nylon, and other synthetic polymers: So, it is not a surprise that amides are the least reactive carboxylic acid derivatives in nucleophilic acyl substitution reactions.
What is an amide?
Definition of amide. Amides (RCONH 2) are functional group where carbonyl group attached to a amine group. In simple amides nitrogen attached with two hydrogen atoms. And in complex amides nitrogen attached with one or two aliphatic or aromatic group replacing the hydrogen atom.
How are amides formed?
Synthesis of amides. Amides are usually formed from the reaction between an amine and a carboxylic acid. This is an condensation reaction and water molecule is removed during the reaction. This reaction is occurred in few steps: Step 1: A nucleophilic nitrogen attacks the carbonyl carbon center of carboxylic acid and oxygen atom pulls ...
Why do amides have no basicity?
Unlike amines, amides show no measurable basicity because of the presence of more electronegative atom near to it. The lone pair of electron on the nitrogen atom is pulled and delocalized through the oxygen, carbon and nitrogen atoms. As a result there are no available electrons on nitrogen to show the basicity of the compound.
Why are low amides soluble in water?
Low amides are soluble in water because they can have hydrogen bond with water molecules. Amides both act as a hydrogen bond donor and acceptor. The nitrogen and oxygen atom act as acceptor and the hydrogens attached with nitrogen atom act as donor.
Why are amides unreactive?
Amide breaks down to its corresponding amine and carboxylic acid. Amides are comparatively unreactive because of delocalization of electrons. However an electronegative atom can attack on the carbonyl carbon and break the pi-bond. The tetrahedral intermediate eventually breaks to form amine and carboxylic acid under acidic condition.
Which atoms are on the same plane?
The electrons of three p orbitals on three atoms oxygen, carbon and nitrogen are are on the same plane and delocalized. Normally the name starts with the name of long chain aliphatic and ends with -amide. Although the melting point of methanamide is liquid but the other higher carbon chain amides are solid at room temperature.
What is the name of the first compound?
As for example, the name of the first compound is ethanamide. When aliphatic or aromatic group is attached to the nitrogen atom, the name of these groups are placed at first, like in the second one, the name is N, N -dimethylmethanamide.
What is an amide?
An amide is a functional group containing a carbonyl group linked to a nitrogen atom or any compound containing the amide functional group. Amides are derived from carboxylic acid and an amine.
What are some examples of amides?
Examples of Amides. Examples of amides include carboxamides, sulfonamides, and phosphoramides. Nylon is a polyamide. Several drugs are amides, including LCD, penicillin, and paracetamol.
What are amides used for?
Amides may be used to form resilient structural materials (e.g., nylon, Kevlar). Dimethylformamide is an important organic solvent. Plants produce amides for a variety of functions. Amides are found in many drugs.
What is an amide?
Amide, any member of either of two classes of nitrogen-containing compounds related to ammonia and amines. The two classes are covalent amides, which are neutral or very weakly acidic substances, and ionic amides, which are strongly alkaline compounds. Amide, any member of either of two classes of nitrogen-containing compounds related ...
What is an ionic amide?
Ionic, or saltlike, amides are strongly alkaline compounds ordinarily made by treating ammonia, an amine, or a covalent amide with a reactive metal such as sodium.
How are simple amides prepared?
Simple amides ordinarily are prepared by reaction of acids or acid halides with ammonia or amines. They can also be produced by the reaction of water with nitriles. Get a Britannica Premium subscription and gain access to exclusive content. Subscribe Now.
What are amides used for?
Among the amides of commercial importance are acetamide, also called ethanamide (CH3CONH2) and dimethylformamide HCON(CH3)2, which are used as solvents, the sulfa drugs, and the nylons. Ureaor carbamide [CO(NH2)2] is a crystalline compoundthat is formed as the end product of the metabolism of protein and excreted in the urine of mammals. It is synthesized in large quantities from ammonia and carbon dioxidefor use in fertilizers, in animal feed, and in manufacturing a class of polymers known as urea-formaldehyde resins, used in making plastics.
What is the reaction of covalent amides?
The characteristic reaction of covalent amides is hydrolysis(a chemical reactionwith water), by which they are converted to acids and amines; this reaction ordinarily is slow unless it is catalyzed by a strong acid, an alkali, or an enzyme. Amides also can be dehydrated to nitriles.
What is the reducing agent that transforms amides into amines?
The powerful reducing agent lithium aluminum hydride transforms amides into amines. Reaction of amides with acid chlorides or anhydrides produces imides, which are compounds with two carbonyl (CO) groups attached to the same nitrogenatom.
Which group of compounds is derived from carboxylic acids?
The carboxamides (R′CONR2), which are derived from carboxylic acids (R′COOH), are the most important group. Sulfonamides (RSO2NR2) are similarly related to the sulfonic acids (RSO3H). Ionic, or saltlike, amides are strongly alkaline compounds ordinarily made by treating ammonia, an amine, or a covalent amide with a reactive metal such as sodium.

Covalent Amides
Amide Nomenclature
Production of Amide
Types of Amides
Amide Functional Group
Amide Structure
Basicity
Key Points of Amide
- A nitrogen atom is bonded to a carbonyl carbon atom in the typical structure of amides.
- The acid amide formula or the amide group formula is CO-NH.
- An amide's functional group is as follows:
Covalent Amides
Amide Structure
Amide Nomenclature
Production of Amide
Types of Amides
Chemical Properties of Amides
Basicity
Applications and Occurrence
Things to Remember
- An amine is a nitrogen-containing compound that can be divided into two groups: ammonia and amines.
- In the usual structure of amides, a nitrogen atom is linked to a carbonyl carbon atom.
- CO-NH is the acid amide formula, often known as the amide group formula.
- In the nomenclature for amides, the -ic acid of the common name or the -oic ending of the IU…
- An amine is a nitrogen-containing compound that can be divided into two groups: ammonia and amines.
- In the usual structure of amides, a nitrogen atom is linked to a carbonyl carbon atom.
- CO-NH is the acid amide formula, often known as the amide group formula.
- In the nomenclature for amides, the -ic acid of the common name or the -oic ending of the IUPAC for the equivalent carboxylic acid is substituted with -amide.