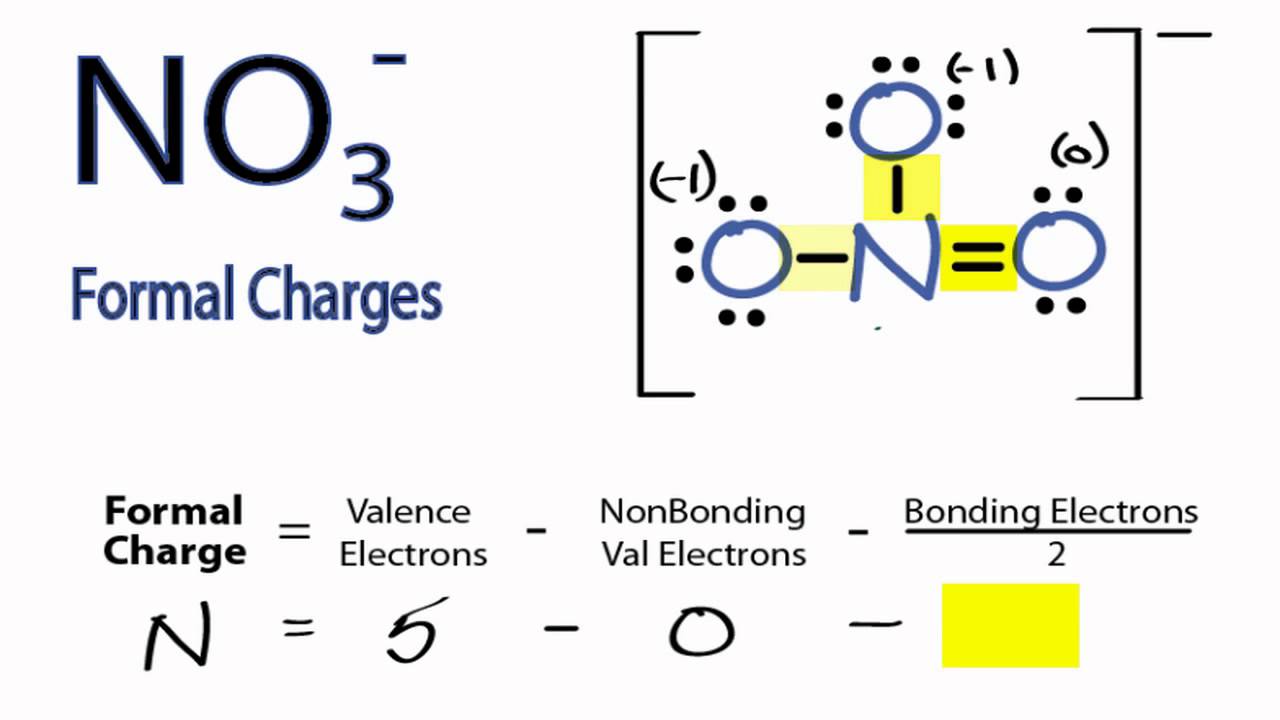
What is the Lewis structure of NO3 ion?
In the Lewis structure of NO3 – ion, the outer atoms are oxygen atoms. So now, you have to complete the octet on these oxygen atoms (because oxygen requires 8 electrons to have a complete outer shell). Now, you can see in the above image that both the oxygen atoms form an octet.
How many atoms of nitrogen are there in NO3?
In the ion NO3, there is 1 atom of nitrogen and 3 atoms of oxygen. It also has one negative charge. 2. Nitrogen and oxygen belong to periods 5A and 6A groups respectively in the periodic table.
How many lone pairs are there in NO3?
In NO 3– we can see that the central atom is bonded with three oxygen atoms and there are no lone pairs. If we check the Lewis structure further then one of the nitrogen-oxygen bonds is a double bond and two are single bonds. During bonding, nitrogen’s three sp2 orbitals overlap with one s orbital of the oxygen atom.
What is the molecular geometry and bond angles of NO3?
The central atom nitrogen is bonded with three oxygen atoms and there are no lone pairs present. Nitrogen’s three sp2 orbitals overlap with one s orbital of the oxygen atom. The p orbital of nitrogen forms a double bond with three oxygen atoms. NO 3– Molecular Geometry And Bond Angles
How do you draw the structure of NO3 negative?
0:022:12Lewis Dot Structure of NO3- (Nitrate Ion) - YouTubeYouTubeStart of suggested clipEnd of suggested clipAll right matron can't violate the octet rule it's only in period two so to complete this structureMoreAll right matron can't violate the octet rule it's only in period two so to complete this structure going to put brackets around it and a negative charge. Now.
What is the Vsepr structure of NO3 −?
0:001:49NO3- Molecular Geometry / Shape and Bond Angles - YouTubeYouTubeStart of suggested clipEnd of suggested clipWe have one of those x that's the number of atoms bonded to this central nitrogen we have one twoMoreWe have one of those x that's the number of atoms bonded to this central nitrogen we have one two three oxygens bonded to it. And that's the number of non-bonded electron pairs on that nitrogen.
What is the resonance structure of NO3 negative?
Resonance structures of NO3- ion When we draw resonance structures, we convert lone pairs to bonds and bonds to lone pairs when it is possible. In lewis structure of NO3- ion, there are three lone pairs (in the last shell) in two oxygen atom and that oxygen atoms. Also, those two oxygen atoms has a -1 charge.
What is NO3 negative?
-1Nitrate is a nitrogen oxoanion formed by loss of a proton from nitric acid. Principal species present at pH 7.3. It is a nitrogen oxoanion, a member of reactive nitrogen species and a monovalent inorganic anion....3.1Computed Properties.Property NameProperty ValueReferenceFormal Charge-1Computed by PubChem17 more rows
What is the bond order for no − 3?
1.33Thus, the bond order of N-O bonds in \[NO_3^ - \] is 1.33.
Which is the correct Lewis structure for no3 − no3 −?
0:001:58How to Draw the Lewis Structure for NO3- - YouTubeYouTubeStart of suggested clipEnd of suggested clipThis is the no3 minus lewis structure the nitrate ion nitrogen has five valence electrons oxygen hasMoreThis is the no3 minus lewis structure the nitrate ion nitrogen has five valence electrons oxygen has six we have three oxygens.
How many resonance structures are possible for NO3 −?
three resonance structuresThe nitrate ion (NO−3) is a resonance hybrid of three resonance structures.
How many resonance structures will no − 3 have?
Explanation: The nitrate ion has three resonance contributors.
Why does NO3 have a negative charge?
The oxygens which have double-bonds have a shared or owned eight electrons, so they are considered neutral. Finally, the single bound oxygen atoms have nine electrons linked with them, and they have a negative charge overall. This means the nitrate ion has an overall charge of -1.
Is NO3 trigonal planar?
In essence, nitrate has 3 electron domains and no lone pairs. Therefore, NO3– molecular geometry is slightly bent and is trigonal planar.
What is the electron dot structure of NO3?
There are one nitrogen atom and three oxygen atoms in the nitrate ion. Also there is a -1 charge on the nitrate ion. Nitrogen and oxygen are located at VA and VIA groups respectively in the periodic table. So nitrogen has five electrons in its valence shell.
How many electron dots are in the Lewis structure of NO3 −?
24 valence electronsThere are 24 valence electrons available for the Lewis structure for NO3-.
What is the shape of the molecule NO3?
The trigonal planar shape of the NO3 molecule creates symmetry across the bonds NO bonds and as a result, the three dipoles created by NO bonds get canceled by each other, and the overall dipole of NO3 is zero. Therefore, the NO3 is a non-polar molecule.
What is the point group of NO3?
It has been observed that in going from a nitrate ion (point group n3h sym- metry) to a covalent nitrate (point group Czv symmetry), a lowering of symmetry occurs (9).
Is NO3 polar or nonpolar molecule?
It is non-polar because it has a trigonal planar structure and the symmetry means that there is an even distribution of electron charge density over the three N−O bond.
Is NO3 planar or nonplanar?
Hint:The molecule is planar and non-planar depending upon the shape of a molecule if in a molecule the atom arranges itself around the central molecules so they exist on a single two-dimensional plane molecule is planar....Total number of electron pairsShape2Linear3Trigonal planar4Tetrahedral5Trigonal bipyramidal2 more rows
Is nitrate a monovalent or a conjugate?
Nitrate is a nitrogen oxoanion formed by loss of a proton from nitric acid. Principal species present at pH 7.3. It is a nitrogen oxoanion, a member of reactive nitrogen species and a monovalent inorganic anion. It is a conjugate base of a nitric acid.
Is nitrate an inorganic or aqueous solution?
It is a nitrogen oxoanion, a member of reactive nitrogen species and a monovalent inorganic anion. It is a conjugate base of a nitric acid. Nitrates, inorganic, aqueous solution, n.o.s. is a liquid which is readily ignited when in contact with organic materials.
What is NO3 nitrate?
Facts About NO3 Nitrate. Nitrate is a salt comprised of nitric acid, and different alcohols, as well as esters, are sometimes referred to as nitric acid. Nitrate ions have a molecular mass of 63, and they are comprised of one nitrogen atom linked with three exactly the same oxygen atoms.
How many electrons does a nitrogen atom have?
Since the nitrogen atom has only 6 electrons total, it, therefore, has a formal positive charge. The oxygens which have double-bonds have a shared or owned eight electrons, so they are considered neutral. Finally, the single bound oxygen atoms have nine electrons linked with them, and they have a negative charge overall. This means the nitrate ion has an overall charge of -1.
What happens when nitrates are combined with other molecules?
In cases where nitrates are combined with other molecules, like when sodium combines with nitrate to form sodium nitrate or NaNo3, the single electron found in sodium’s outer shell will be transferred over to N03, which makes it a nitrate ion and ends up making it have a -1 charge.
Why does nitrogen have a positive charge?
The nitrogen has a positive charge because it has 4 bonding electrons – 2 from the oxygen double bond and 1 from each of the N – O bonds. The inner core of the electrons is associated with just 6 electrons instead of the 7 that are needed for electrical neutrality, meaning it has a positive charge overall.
What is the charge of nitrates?
Nitrate, chemical formula NO3, has a chemical charge of -1. Ion nitrates have a negative one formal charge. You may be wondering why this is the case. Why isn’t the full charge of N03 -9?
What is the nitro functional group?
Organic compounds that contain the nitro functional group are referred to as nitro compounds . Nitrate ions can be quite dangerous and toxic, and it affects humans through the process of nitrate toxicosis – a condition where iron atoms found in the blood’s hemoglobin are oxidized and become unable to carry oxygen.
Where are nitrates found?
Nitrates are rather commonly found in large deposits, especially deposits of nitratine. These nitratine deposits act as major fonts of sodium nitrate, also known as Chile Saltpeter. Nitrites are made by bacteria, and the nitrate compounds that this bacteria produces were historically used for gunpowder and produced by fermentation. Nitrates are also used in fertilizer because of their biodegradable properties and their high solubility. The primary nitrate fertilizers include calcium salts, potassium, ammonium, and sodium. Nitric acid is also produced during lightning strikes, owing to the interactions between water vapor and nitrogen dioxide.
How many atoms are in a nitrate ion?
There are one nitrogen atom and three oxygen atoms in the nitrate ion. Also there is a -1 charge on the nitrate ion.
How many lone pairs does an oxygen atom have?
Start to mark those nine valence electrons pairs on outside atoms (oxygen atoms) as lone pairs. One oxygen atom will take three lone pairs following the octal rule (oxygen and nitrogen atoms cannot keep more than eight electrons in their valence shells). All nine valence electrons pairs (9) are spent when lone pairs are marked on oxygen atoms.
Is there an ine valence electron pair for nitrogen?
Therefore, there is no ine valence electrons pairs to mark on nitrogen atom.
Is there a double bond between nitrogen and oxygen?
Now there is a double bond between nitrogen and one oxygen atom. There are also two single bonds (N-O) with nitrogen atom and other oxygen atoms.
Does nitrogen have electrons?
No electrons pairs exist on nitrogen atom. But, on nitrogen atom, there is a +1 charge. Around the nitrogen atom, there are two single bonds and double bond.
How many electrons does nitrogen have?
Nitrogen has a total of 7 electrons - two in its inner shell and 5 in its second shell. So by the reasoning above, you'd think that nitrogen has room for 3 more electrons in its second shell. And indeed it does. And some compounds, such as lithium nitride, Li3N, follow this pattern.
What is the difference between nitrogen and oxygen?
The difference is in the nitrogen atoms in each the oxidation number is different. In NO with oxygen having -2 as its oxidation number (one of the major rules of oxidation numbering) the nitrogen has to have +2 as its oxidation number.
What is the oxidation number of oxygen?
Oxygen in compounds has an oxidation number of -2, unless it conflicts with Rule #1 (e.g. OF2). ... Subsequent rules all have the caveat that they don't conflict with lower-numbered rules. Nitrogen has an oxidation state of -3 unless that conflicts with lower-numbered rules.
Which atom gives away its outer shell electrons to the three oxygen atoms?
A sodium atom gives its single outer shell electron to a chlorine atom, forming table salt. So nitrogen gives away its five outer shell electrons to the three oxygen atoms, because the oxygen atoms pull more strongly on the nitrogen's outer electrons than nitrogen's own nucleus does.
Is oxygen electronegative?
Technically, we say that oxygen is highly electronegative). Yes, there is some logic behind it, but your excellent question shows that the answer is not obvious. Looking at oxygen first, each oxygen atom has 2 electrons in its inner shell, and 6 in its second shell.
Is HNO3 a molecule?
The definition of a molecule includes the words electrically neutral. HNO3 is a molecule, NaNO3 is a molecule, but NO3- is a polyatomic ion. Now, the ion has an overall charge, or net charge of -1, not a formal charge. The oxygen atom connected by a single bond has a formal charge of -1.
How many oxygen atoms are in nitrogen?
The central atom nitrogen is bonded with three oxygen atoms and there are no lone pairs present. Nitrogen’s three sp2 orbitals overlap with one s orbital of the oxygen atom. The p orbital of nitrogen forms a double bond with three oxygen atoms.
How many pairs of electrons are shared between the p orbital of nitrogen and the oxygen atom?
As for the p orbital of nitrogen, it forms a double bond with three oxygen atoms where three pairs of electrons are shared between the p orbital of the nitrogen and one p orbital of each oxygen atoms.
What is the Hybridization of Nitrate?
The easiest way to determine the hybridization of nitrate is by drawing the Lewis structure. After drawing the diagram, we need to count the number of electron pairs and the bonds present in the central nitrogen atom. In NO 3– we can see that the central atom is bonded with three oxygen atoms and there are no lone pairs. If we check the Lewis structure further then one of the nitrogen-oxygen bonds is a double bond and two are single bonds.
Is nitrate a trigonal plane?
In essence, nitrate has 3 electron domains and no lone pairs. Therefore, NO 3– molecular geometry is slightly bent and is trigonal planar. The bond angle is 120 o.
