
What is vanillin and where does it come from?
What is vanillin and where does it come from? Vanillin is the main chemical compound of the extract of the vanilla bean. Nowadays, vanillin is mainly used as a flavouring agent, usually in sweet foods such as ice cream and chocolate. Did you know that 99% of the vanillin today does not come from the vanilla beans but is produced synthetically?
What functional groups are present in vanillin?
Vanillin is an organic compound with the molecular formula C8H8O3. It is a phenolic aldehyde. Its functional groups include aldehyde, hydroxyl, and ether. It is the primary component of the extract of the vanilla bean. Read complete answer here.
What is vanillin used for?
Vanillin is used in the preparation of pharmaceutical drugs for Parkinson’s disease, hypertension and many other drugs. Vanillin is added to cosmetics to make it smell better and it is added to soap as well to make it smell better. Vanillin is used in feed industry as animal nutrition.
What is the empirical formula of vanillin?
Vanillin, the dominant flavoring in vanilla, contains C, H, and O. When 1.05 g of this substance is completely combusted, 2.43 g of CO2 and 0.50 g of H2O are produced. What is the empirical formula of vanillin? Students also viewed these Chemical Engineering questions Vanillin, C8H8O3, occurs naturally in vanilla extract and is used as a
See more

What does vanillin look like?
Vanillin appears as white or very slightly yellow needles. WHITE-TO-YELLOW CRYSTALLINE POWDER WITH CHARACTERISTIC ODOUR.
What functional groups are in vanillin?
Vanillin, as its name implies, is the major flavor component of vanilla. The three oxygen atoms in this small aromatic compound are in different functional groups: alcohol, aldehyde, and ether. It is a white crystalline solid with a melting point of 81–83 ºC.
What is the chemical name for vanillin?
ortho-Vanillin. 2-Hydroxy-5-methoxybenzaldehyde. 2-Hydroxy-4-methoxybenzaldehyde.
Is vanillin a mixture?
In contrast to vanilla extract, vanillin is a single chemical compound which can be synthesized either chemically or biologically. Synthetic vanillin is most widely used due to its low cost.
What type of bond is vanillin?
The vanillin molecule contains a total of 19 bond(s) There are 11 non-H bond(s), 7 multiple bond(s), 2 rotatable bond(s), 1 double bond(s), 6 aromatic bond(s), 1 six-membered ring(s), 1 aldehyde(s) (aromatic), 1 aromatic hydroxyl(s) and 1 ether(s) (aromatic).
Is vanillin a carbohydrate?
COMPOSITION: Pure crystallized vanillin 99.6%. NUTRITION INFORMATION PER 100g: 0 kcal/100g. Carbohydrates: 0% (0% of them are polyols).
What is the difference between vanillin and vanilla?
Vanillin is the naturally occurring chemical compound that we recognize as the primary aroma and taste of vanilla. And although real vanilla extract is made up of vanillin (plus lesser compounds that add to its varying levels of complexity), sometimes the vanillin is all you need to spark that familiar flavor.
How is vanillin formed?
Other studies have suggested that vanillin is formed from L-phenylalanine via the monomeric lignin precursors: cinnamic acid, p-coumaric acid, caffeic acid and ferulic acid, involving phenylalanine ammonia lyase (PAL), hydroxylations, an O-methylation and finally a chain-shortening reaction.
Is vanillin an alcohol?
Vanillyl alcohol is derived from vanillin....Vanillyl alcohol.NamesOther names 3-Methoxy-4-hydroxybenzyl alcohol 4-Hydroxy-3-methoxybenzenemethanol 4-Hydroxy-3-methoxybenzyl alcohol Vanillic alcohol Vanillin alcoholIdentifiersCAS Number498-00-0ChEBICHEBI:1835318 more rows
Is vanillin a sugar?
The sugar product is called vanillin zucker, which is a variation on vanilla sugar that uses vanillin instead of whole vanilla beans or vanilla bean seeds. Vanillin is the primary substance of natural vanilla bean extract, and it is cheaper than vanilla beans....Ingredients.Nutrition Facts (per serving)0gProtein3 more rows•May 5, 2021
Why is vanillin soluble in water?
Molecules containing only carbon and hydrogen are mostly insoluble in water. The oxygen-containing groups attached to the ring in vanillin can form strong hydrogen bonds with water, making it water soluble (about a gram of vanillin can be dissolved in 100 mL of cold water).
Does vanillin dissolve in water?
Vanillin is soluble in water,ethyl alcohol, pyridine ,glycerol,oils and aqueous alkali hydroxides.
Does vanillin contain a phenol functional group?
Vanillin has functional groups aldehyde, ether, and phenol.
What functional groups are in cinnamaldehyde?
Cinnamaldehyde is a representative compound that contains two unsaturated functional groups of aldehyde and carbon–carbon double bond.
Which functional group of vanillin is mostly responsible for the slight water solubility?
The oxygen-containing groups attached to the ring in vanillin can form strong hydrogen bonds with water, making it water soluble (about a gram of vanillin can be dissolved in 100 mL of cold water).
What functional groups are in pyruvic acid?
Pyruvic acid (CH3COCOOH) is the simplest of the alpha-keto acids, with a carboxylic acid and a ketone functional group.
What enzyme catalyzes hydration of the double bond in ferulic acid?
Caffeic acid then undergoes methylation by caffeic acid O- methyltransferase (COMT) to give ferulic acid. Finally, vanillin synthase hydratase/lyase (vp/VAN) catalyzes hydration of the double bond in ferulic acid followed by a retro-aldol elimination to afford vanillin.
What is the pathway of vanillin biosynthesis?
Vanillin biosynthesis is generally agreed to be part of the phenylpropanoid pathway starting with L -phenylalanine, which is deaminated by phenylalanine ammonia lyase (PAL) to form t- cinnamic acid. The para position of the ring is then hydroxylated by the cytochrome P450 enzyme cinnamate 4-hydroxylase (C4H/P450) to create p - coumaric acid.
What is the effect of heat treatment on vanillin?
In other foods, heat treatment generates vanillin from other compounds. In this way, vanillin contributes to the flavor and aroma of coffee, maple syrup, and whole-grain products, including corn tortillas and oatmeal.
What is the flavor of vanilla?
Vanillin is most prominent as the principal flavor and aroma compound in vanilla. Cured vanilla pods contain about 2% by dry weight vanillin; on cured pods of high quality, relatively pure vanillin may be visible as a white dust or "frost" on the exterior of the pod.
What is vanillin made of?
Today, approximately 15% of the world's production of vanillin is still made from lignin wastes, while approximately 85% synthesized in a two-step process from the petrochemical precursors guaiacol and glyoxylic acid. Beginning in 2000, Rhodia began marketing biosynthetic vanillin prepared by the action of microorganisms on ferulic acid extracted ...
What is the compound used to make vanillin?
The first commercial synthesis of vanillin began with the more readily available natural compound eugenol (4-allyl-2-methoxyphenol).
How long do pods sweat?
Then, for 1–2 weeks, the pods are alternately sunned and sweated: during the day they are laid out in the sun, and each night wrapped in cloth and packed in airtight boxes to sweat. During this process, the pods become dark brown, and enzymes in the pod release vanillin as the free molecule.
How to calculate vanillin weight?
The molecular weight of vanillin is available in molecular weight page of vanillin, which is calculated as the sum of the atomic weights of each constituent element multiplied by the number of atoms of that element in the molecular formula.
What is a SDF file?
The structure data file (SDF/MOL File) of vanillin is available for download in the SDF page of vanillin, which provides the information about the atoms, bonds, connectivity and coordinates of vanillin. The vanillin structure data file can be imported to most of the cheminformatics software systems and applications.
What is the molecular formula of vanillin?
The molecular formula of vanillin is available in chemical formula page of vanillin, which identifies each constituent element by its chemical symbol and indicates the proportionate number of atoms of each element.
Why is the radius of the spheres smaller than the rod lengths?
The radius of the spheres is therefore smaller than the rod lengths in order to provide a clearer view of the atoms and bonds throughout the chemical structure model of vanillin.
What is the 2D chemical structure of vanillin?
The 2D chemical structure image of vanillin is also called skeletal formula, which is the standard notation for organic molecules. The carbon atoms in the chemical structure of vanillin are implied to be located at the corner (s) and hydrogen atoms attached to carbon atoms are not indicated – each carbon atom is considered to be associated with enough hydrogen atoms to provide the carbon atom with four bonds.
What is the SMILES string of vanillin?
The SMILES string of vanillin is COc1cc (C=O)ccc1O , which can be can be imported by most molecule editors for conversion back into two-dimensional drawings or three-dimensional models of the vanillin.
Where are the carbon atoms in vanillin?
The carbon atoms in the chemical structure of vanillin are implied to be located at the corner (s) and hydrogen atoms attached to carbon atoms are not indicated – each carbon atom is considered to be associated with enough hydrogen atoms to provide the carbon atom with four bonds. The 3D chemical structure image of vanillin is based on ...
How much vanillin is in vanilla beans?
We’ll use this term for the vanillin naturally occurring in the vanilla bean pod. The highest quality beans contain about 2 percent vanillin.
How are benzene rings formed?
Benzene rings also can be created through chemical reactions from base materials that contain at least six carbons. Vanillin is an aldehyde, which means it has a group of carbons double-bonded to an oxygen atom and single bonded to a hydrogen atom. Shown below is a basic aldehyde structure. (The R represents any carbon grouping; for vanillin, this would be the benzene ring.) Vanillin also has two more oxygen groups opposite to the aldehyde.
What is the ring in vanilla?
Like most flavor components, pure or synthetic, vanillin is a six carbon ring, called benzene , depicted as a hexagon with alternating dashes. The benzene ring is very prominent in nature, and so has many sources besides vanilla.
Where is vanillin found?
Pure vanillin is exclusively found in vanilla beans and is never processed out of the bean pods. The value of pure vanilla comes not from the vanillin alone, but from the delicate complexity of over 400 known flavor components coming together in a unique flavor that simply cannot be duplicated. Synthesizing vanillin from vanilla beans would be like isolating a single component of an expensive and complex red wine and throwing the rest down the sink.
Is vanillin a chemical?
Synthetic vanillin is usually derived from the closest and cheapest chemical structure occurring in nature. Historically, lignin, a byproduct of the paper industry, was used as a starting block. As you can see in the structure of lignin below, it shares the benzene group and the two oxygen groups. Only one reaction is needed to add the characteristic aldehyde group.
Is vanillin a natural substance?
A new vanillin is now on the market that is labeled natural vanillin. This vanillin is derived from a biosynthetic reaction involving fermentation of rice derivatives or sugar. It is labeled natural vanillin because it uses a natural chemical reaction — fermentation — to transform the substrate into vanillin. This is a fairly new method used by just a few companies and so is about 10 times more expensive than synthetically derived vanillin.
Is vanillin an artificial ingredient?
There’s a lot of confusion about vanillin, because it is often thought of as an artificial ingredient. In fact it’s the most prominent flavor component of vanilla beans.
What is vanilla extract?
Vanillin is the primary chemical component of the extract of the vanilla bean. Synthetic vanillin is used as a flavoring agent in foods, beverages, and pharmaceuticals. Natural vanilla extract is a mixture of several hundred different compounds in addition to vanillin. Artificial vanilla flavoring is a solution of pure vanillin, usually of synthetic origin. The largest single use of vanillin is as a flavoring, usually in sweet foods. The ice cream and chocolate industries together comprise 75% of the market for vanillin as a flavoring, with smaller amounts being used in confections and baked goods.
Where does vanilla come from?
Natural vanillin is extracted from the seed pods of Vanilla planifola, a vining orchid native to Mexico, but now grown in tropical areas around the globe. Madagascar is presently the largest producer of natural vanillin. As harvested, the green seed pods contain vanillin in the form of its ?-D-glycoside; the green pods do not have the flavor or odor of vanilla. After being harvested, their flavor is developed by a months-long curing process.
What is vanillin made of?
Despite eugenol being a slightly cheaper way to make vanillin, development has continued and nowadays most of the vanillin is not made from eugenol anymore, instead it is made from lignin and guaiacol. The image below shows an illustration of how lignin could look like ( source ).
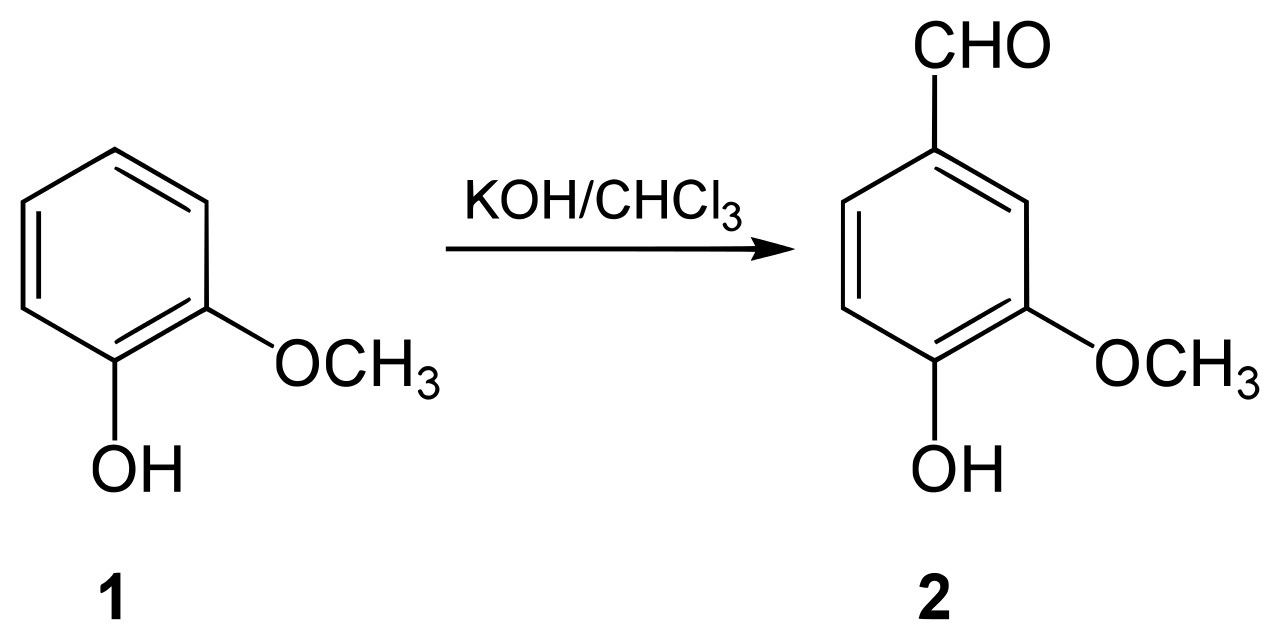
How to make vanillin?
The first is to find a more common molecule that already exists in nature and transform it into vanilla using various chemical reactions. The other method uses yeasts to make vanillin molecules from pretty different molecules. 1.
What is the molecule that makes vanilla?
Vanillin molecule. Pure vanilla originates from vanilla beans and is a complex mixture of flavour molecules. However, there is one molecule that is mostly associated with vanilla: vanillin. It has a huge contribution to natural vanilla and for many of you, vanillin might actually be what you think of when you think of vanilla.
What happens when you convert eugenol to vanillin?
When converting eugenol to vanillin two chemical process steps have to take place: Isomerization of the eugenol: in other words, the double bond of the eugenol will move from the top to one position lower. Oxidation of the isoeugenol, thus adding that oxygen group.
What are the natural processes used to make vanillin?
A comming route that various companies look into for making natural vanillin though is the use of micro organisms. Certain yeasts, fungi and bacteria can be used to make vanillin from other (naturally occuring) molecules. Several processes are already used to make vanillin with the use of micro organisms.
What is vanilla in food?
Comparing processes. flavour, spices & herbs. Vanilla is one of the most common and popular flavours in food. Are there ice cream shops that do not sell a vanilla ice cream? There are several ways to make food taste like vanilla. The most straightforward one is to use the original source: natural vanilla which is a pod that grows on ...
What is the structure of eugenol?
Eugenol can be extracted from spices such as cinnamon, nutmeg and cloves. Its structure is very similar to that of vanillin as you can see in the structure shown below ( source ). The only difference sits in the top branch where it lacks the oxygen (O) group and instead has a longer carbon tail.
What Is Vanillin?
Vanillin is a vanilla extract alternative made from wood pulp. It is commonly used to reduce production costs. And, unlike real vanilla extract which is produced from real vanilla beans, vanillin is synthetic and may be produced using petrochemicals and byproducts from the paper industry. Unfortunately, because it's cheap, the stuff is everywhere. Vanilla flavored over-the-counter medicines, beverages, and cookies are all places you're likely to find vanillin.
Is vanilla bean safe to eat?
Vanillin may not be one of the most dangerous food additives, but it certainly won’t provide you the benefits obtained from pure vanilla. If you purchase processed foods or beverages, always choose organic. Most organic cookies, while still not the most healthy food on the planet, often contain pure vanilla. When baking, always choose organic vanilla beans or organic vanilla extract over artificial vanilla. Your health — and your taste buds — will thank you.
Is vanilla extract good for you?
Vanilla in its pure form has a few health benefits. Real vanilla extract contains a number of antioxidant compounds, at least according to one study. [ 1] . Interestingly enough, this same study claims that vanillin contains a much lower amount of protective activity. Vanilla extract may also be helpful for fighting bacteria.
Is vanillin a toxic food additive?
Don't get too frightened, vanillin isn't one of the most toxic food additives you'll find and in fact usually won't trigger much more than a headache or allergic reaction in sensitive folks. Usually, switching from artificial vanilla extract to pure vanilla extract is all that is needed to avoid issues.
What does the reviewed and approved seal mean?
Additionally, the Reviewed and Approved seal signifies that our scientific board of experts has double-checked this article for accuracy. You can feel confident in knowing that the information within this article is sound.
Is there a vanilla flavoring?
There is, however, a common artificial vanilla flavoring called vanillin that is produced as a way to get "the same thing" at a reduced cost. Unfortunately, vanillin is lacking in flavor and consumption of this artificial enhancer may not be a great idea.
Is vanilla bean inferior to vanilla extract?
Many connoisseurs of the vanilla bean claim vanillin to be an inferior product to pure vanilla extract anyway. If you're making an attempt to eat quality food, you probably won't encounter much vanillin anyway.
What is vanillin used for?
Vanillin can also be used for rubbers and plastics as well. The manufacturing process of tobaccouse need vanillin as a flavouring in cigarettes. Vanillin can be added to the tobacco, cigarette paper or filter. Vanillin masks the harshness of tobacco smoke, making smoking easier.
Why is vanillin added to soap?
Vanillin is added to cosmetics to make it smell better and it is added to soap as well to make it smell better.
Is synthetic vanillin a natural extract?
Synthetic vanillin is now used more often than natural vanilla extract as a flavoring agent in foods, beverages and pharmaceuticals. The application and use such as in ice cream, desserts, baked goods, and beverages. Vanillin uses as follows:
Is vanillin a flavoring agent?
Vanillin Natural and Vanillin synthetic is used as flavoring. Vanillin Natural and Vanillin synthetic used in Food, Beverage, Pharmaceutical, Health & Personal care products, Agriculture/Animal Feed/Poultry. Synthetic vanillin is now used more often than natural vanilla extract as a flavoring agent in foods, beverages and pharmaceuticals.
Overview
History
The oldest possible evidence of vanilla exploitation in the Old World dates from the second millennium BCE.
Vanilla beans, called tlilxochitl, were discovered and cultivated as a flavoring for beverages by native Mesoamerican peoples, most famously the Totonacs of modern-day Veracruz, Mexico. Since at least the early 15th century, the Aztecs used …
Occurrence
Vanillin is most prominent as the principal flavor and aroma compound in vanilla. Cured vanilla pods contain about 2% by dry weight vanillin; on cured pods of high quality, relatively pure vanillin may be visible as a white dust or "frost" on the exterior of the pod.
It is also found in Leptotes bicolor, a species of orchid native to Paraguay and …
Chemistry
Natural vanillin is extracted from the seed pods of Vanilla planifolia, a vining orchid native to Mexico, but now grown in tropical areas around the globe. Madagascar is presently the largest producer of natural vanillin.
As harvested, the green seed pods contain vanillin in the form of its β-D-glucoside; the green pods do not have the flavor or odor of vanilla.
Uses
The largest use of vanillin is as a flavoring, usually in sweet foods. The ice cream and chocolate industries together comprise 75% of the market for vanillin as a flavoring, with smaller amounts being used in confections and baked goods.
Vanillin is also used in the fragrance industry, in perfumes, and to mask unpleasant odors or tastes in medicines, livestock fodder, and cleaning products. It is also used in the flavor industry, as a v…
Adverse effects
Vanillin can trigger migraine headaches in a small fraction of the people who experience migraines.
Some people have allergic reactions to vanilla. They may be allergic to synthetically produced vanilla but not to natural vanilla, or the other way around, or to both.
Vanilla orchid plants can trigger contact dermatitis, especially among people working in the vanilla trade if they come into contact with the plants sap. An allergic contact dermatitis called …
Vanillin can trigger migraine headaches in a small fraction of the people who experience migraines.
Some people have allergic reactions to vanilla. They may be allergic to synthetically produced vanilla but not to natural vanilla, or the other way around, or to both.
Vanilla orchid plants can trigger contact dermatitis, especially among people working in the vanilla trade if they come into contact with the plants sap. An allergic contact dermatitis called vanillis…
Ecology
Scolytus multistriatus, one of the vectors of the Dutch elm disease, uses vanillin as a signal to find a host tree during oviposition.
See also
• Phenolic compounds in wine
• Other positional isomers:
• Benzaldehyde
• Protocatechuic aldehyde
• Syringaldehyde
Chemical Structure Description
Additional Information For Identifying Vanillin Molecule
- The SMILES string of vanillin is COc1cc(C=O)ccc1O, which can be can be imported by most molecule editors for conversion back into two-dimensional drawings or three-dimensional models of the vanillin.
- The structure data file (SDF/MOL File) of vanillin is available for download in the SDF page of vanillin, which provides the information about the atoms, bonds, connectivity and coordinate…
- The SMILES string of vanillin is COc1cc(C=O)ccc1O, which can be can be imported by most molecule editors for conversion back into two-dimensional drawings or three-dimensional models of the vanillin.
- The structure data file (SDF/MOL File) of vanillin is available for download in the SDF page of vanillin, which provides the information about the atoms, bonds, connectivity and coordinates of vani...
- The molecular formula of vanillin is available in chemical formula page of vanillin, which identifies each constituent element by its chemical symbol and indicates the proportionate number of atoms...
- The molecular weight of vanillin is available in molecular weight page of vanillin, which is cal…
Additional Outstanding Products
- Visit ChemTopia for further professional chemical information on the basis of a comprehensive intelligence networking platformfor experts in the discipline around the globe.
- Need to identify active compounds in your natural products? Then, try SnaPeaks – simply upload your MS/MS data and SnaPeakswill provide what’s in your natural products.
- Conversion of complicated chemical-related units is no longer sophisticated with the aid of U…
- Visit ChemTopia for further professional chemical information on the basis of a comprehensive intelligence networking platformfor experts in the discipline around the globe.
- Need to identify active compounds in your natural products? Then, try SnaPeaks – simply upload your MS/MS data and SnaPeakswill provide what’s in your natural products.
- Conversion of complicated chemical-related units is no longer sophisticated with the aid of UnitPot.UnitPot is a noteworthy web-based scientific unit converter that comes with an intuitive user int...
Vanillin Identification Summary Frequently Asked Questions
- 9338 The contents of this page can freely be shared if cited as follows: Source: Mol-Instincts Chemical Database, Predicted on Quantum. Please hyperlink "Mol-Instincts" to www.molinstincts.com.