How do you calculate the number of protons and neutrons?
Part 1 Part 1 of 2: Calculating Protons, Electrons, and Neutrons
- Get a periodic table of elements. The periodic table is a chart that organizes elements by their atomic structure.
- Find your element on the periodic table. The table orders elements by atomic number and separates them into three main groups: metals, non-metals, and metalloids (semi-metals).
- Locate the element’s atomic number. ...
Do protons have greater masses than neutrons?
Protons and neutrons do not have the same mass. Their masses are quite close, but they are noticeably different. This is the case because the binding energy of a proton or neutron in a nucleus is about 8 MeV (Million Electron Volts) on average and that’s bigger than the proton-neutron mass difference.
What is the actual mass of a proton, an electron and a neutron?
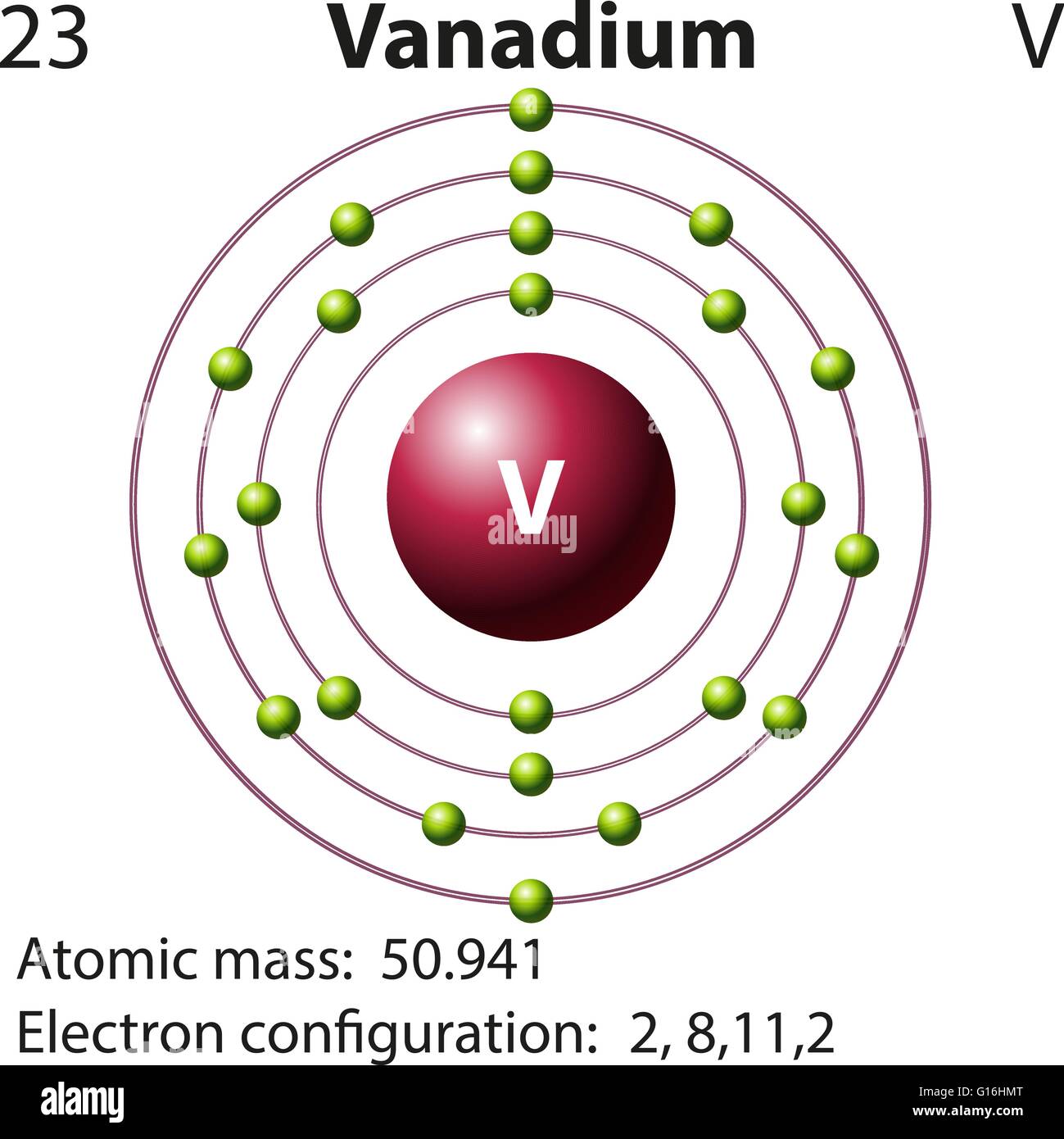
The negative charge of one electron balances the positive charge of one proton. Both protons and neutrons have a mass of 1 , while electrons have almost no mass. The element hydrogen has the simplest atoms, each with just one proton and one electron. The proton forms the nucleus, while the electron orbits around it.
Are protons and neutrons always the same number?
The number of protons in the nucleus of every atom of an element is always the same, but this is not the case with the number of neutrons. Atoms of the same element can have a different number of neutrons. Atoms want to have the same number of neutrons and protons but the number of neutrons can change.
How many protons are in an atom?
What is the particle that is smaller than an atom?
How many isotopes does copper have?
When two elements react to form more than one compound, what happens?
Which element has more than one charge?
Does a compound always contain the same proportion of elements by mass?
See 3 more
About this website

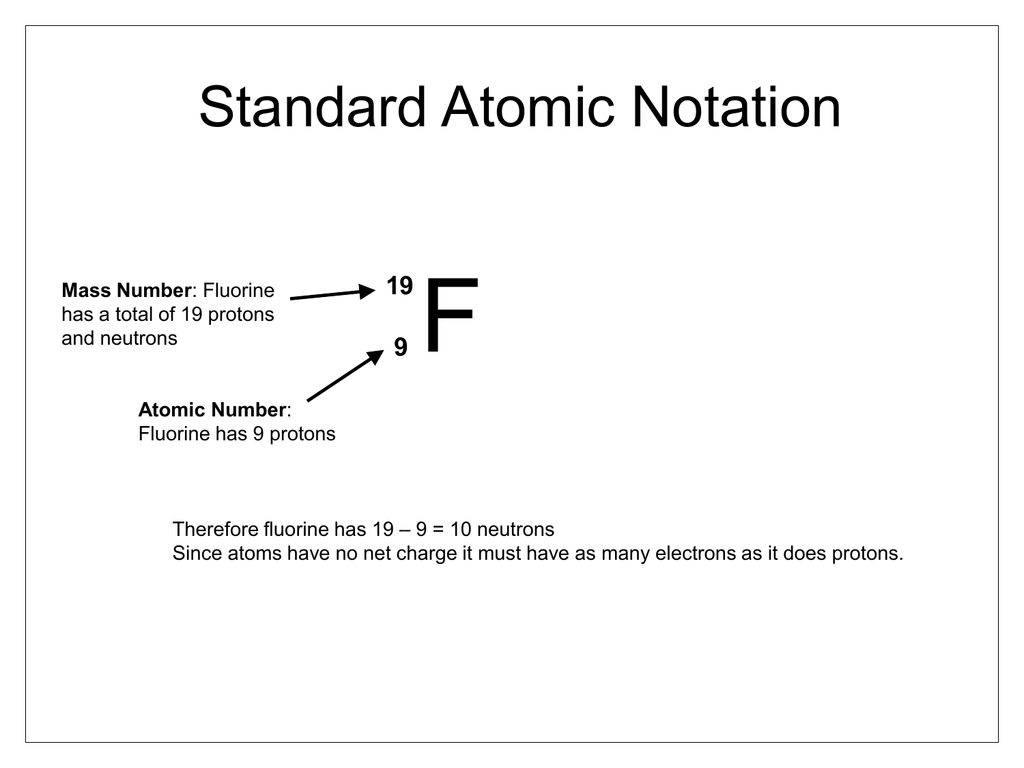
What is the sum of the protons and neutrons in its nucleus?
The sum of the numbers of protons and neutrons in the nucleus is called the mass number and, expressed in amu, is approximately equal to the mass of the atom. An atom is neutral when it contains equal numbers of electrons and protons.
What is the sum of electrons protons and neutrons?
The heaviest isotope of hydrogen is tritium 1T3 with atomic number 1 and mass number 3. It contains 1 electron, 1 proton and 2 neutrons. The sum is 1+1+2=4.
What is the number of neutrons and protons called?
Mass NumberMass Number. The mass number (A) of an atom is the total number of protons and neutrons in its nucleus. The mass of the atom is a unit called the atomic mass unit (amu).
What do you call the sum of the number of protons?
Detailed Solution The Atomic mass or mass number is the sum of the number of protons and neutrons.
What is the sum of protons and electrons?
The number of protons in the nucleus of the atom is equal to the atomic number (Z). The number of electrons in a neutral atom is equal to the number of protons. The mass number of the atom (M) is equal to the sum of the number of protons and neutrons in the nucleus.
What does the atomic mass the sum of?
Together, the number of protons and the number of neutrons determine an element's mass number: mass number = protons + neutrons.
What made up of an atom?
An atom consists of a central nucleus that is surrounded by one or more negatively charged electrons. The nucleus is positively charged and contains one or more relatively heavy particles known as protons and neutrons. Atoms are the basic building blocks of matter.
What is the sum of all the protons electrons and neutrons in an atom of carbon 12?
The sum of the number of protons and neutrons in an atom is called the mass number. For example: Carbon-12 has 12 protons and 12 neutrons. 12+12=24 , so the mass number of C-12 is 24 (no units).
Chapter 2 Part 1 Atomic Structure Flashcards | Quizlet
Study with Quizlet and memorize flashcards containing terms like A 100 g sample comprised of molecule (A) is made up of 57.1 g of oxygen and 42.9 g of carbon. Another 100 g sample comprised of molecule (B) is made up of 72.7 g oxygen and 27.3 g carbon. The ratio of oxygen: carbon mass ratios between the two molecules is 2:1. Which law is demonstrated in this calculation?
quiz 2 chem 100 Flashcards | Quizlet
Study with Quizlet and memorize flashcards containing terms like Label B is pointing to a subatomic particle that is found inside the nucleus (labeled with an A) on an atom. The two subatomic particles in the nucleus are protons and neutrons so label B could be either one., The prefix in subatomic tells us that subatomic particles are:, Which has the smallest mass? and more.
Solved Due Tuesday by 11:59pm 20 ming Question Which of the | Chegg.com
Transcribed image text: Due Tuesday by 11:59pm 20 ming Question Which of the following was Robert A. Millikan able to determine, either from his own work, or by building on the work of J.J. Thomson? Select all that apply. Select all that apply: 0 The existence of an electron The charge of an electron The mass of an electron The model of an atom with a small, positively charged nucleus. le mine ...
How many protons are in an atom?
Write the chemical formula for the atom that has 15 protons and 16 neutrons. Include the correct atomic symbol, mass number, and atomic number. Write the answer in the form XZA where A is the mass number, Z is the atomic number, and X is an elemental symbol. d.
What is the particle that is smaller than an atom?
A)The existence of a small (lighter than an atom), negatively charged fundamental particle called the electron.
How many isotopes does copper have?
Copper exists in two naturally occurring isotopes. The average atomic weight of copper is 63.546 amu. If 69.15% exists as 63Cu with an atomic mass of 62.92, then what is the atomic mass of the second isotope?
When two elements react to form more than one compound, what happens?
When two elements react to form more than one compound, a fixed mass of one element will react with masses of the other element in a ratio of small whole numbers. Law of multiple proportions. ( also, representative elements) element in groups 1,2 and 13-18. Main-group element.
Which element has more than one charge?
B) most have more than one possible charge for cations. Examples: Iron: Fe^2+ and Fe^3+, copper: Cu^+ and Cu^2+, Gold: Au^+ and Au^3+
Does a compound always contain the same proportion of elements by mass?
A given compound will always contain the same proportion of elements by mass.
How many protons are in an atom?
Write the chemical formula for the atom that has 15 protons and 16 neutrons. Include the correct atomic symbol, mass number, and atomic number. Write the answer in the form XZA where A is the mass number, Z is the atomic number, and X is an elemental symbol. d.
What is the particle that is smaller than an atom?
A)The existence of a small (lighter than an atom), negatively charged fundamental particle called the electron.
How many isotopes does copper have?
Copper exists in two naturally occurring isotopes. The average atomic weight of copper is 63.546 amu. If 69.15% exists as 63Cu with an atomic mass of 62.92, then what is the atomic mass of the second isotope?
When two elements react to form more than one compound, what happens?
When two elements react to form more than one compound, a fixed mass of one element will react with masses of the other element in a ratio of small whole numbers. Law of multiple proportions. ( also, representative elements) element in groups 1,2 and 13-18. Main-group element.
Which element has more than one charge?
B) most have more than one possible charge for cations. Examples: Iron: Fe^2+ and Fe^3+, copper: Cu^+ and Cu^2+, Gold: Au^+ and Au^3+
Does a compound always contain the same proportion of elements by mass?
A given compound will always contain the same proportion of elements by mass.