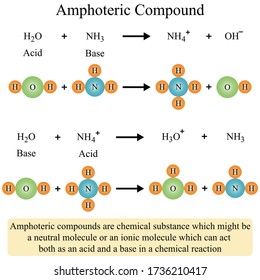
A neutralization reaction can be defined as a chemical reaction in which an acid and base quantitatively react together to form a salt and water as products. In a neutralization reaction, there is a combination of H + ions and OH – ions which form water. A neutralisation reaction is generally an acid-base neutralization reaction.
What two things does a neutralization reaction always produce?
- formic acid, HCOOH
- acetic acid, CH3COOH
- benzoic acid, C6H6COOH
- taurine, C2H7NO3S
What do you observe during neutralization reaction?
In chemistry, neutralization or neutralisation is a chemical reaction in which acid and a base react quantitatively with each other. In a reaction in water, neutralization results in there being no excess of hydrogen or hydroxide ions present in the solution. The pH of the neutralized solution depends on the acid strength of the reactants.
What is the real life example of neutralization?
The following examples will illustrate common neutralization reactions that occur around us. During indigestion, too much of acid is produced inside the stomach resulting in stomach disorder or acidity. In order to retrieve the pain we take bases such as milk of magnesia which contains magnesium hydroxide.
What are some daily life examples of neutralization reactions?
Some household examples of neutralization include:
- Toothpaste neutralizing mouth acid from bacteria
- Compost neutralizes acids in soil
- Vinegar can treat alkaline stings
- Antacids neutralize stomach acid
- Baking soda neutralizes acidic odors
- Acidic conditioner neutralizes alkaline shampoo

What is the process of a neutralization reaction?
A neutralization reaction is when an acid and a base react to form water and a salt and involves the combination of H+ ions and OH- ions to generate water. The neutralization of a strong acid and strong base has a pH equal to 7.
What are the 2 products of a neutralization reaction?
Neutralization is the reaction of an acid and a base, which forms water and a salt.
What are the reactants of neutralization reaction?
Neutralization reactions occur when two reactants, an acid and a base, combine to form the products salt and water.
What type of reaction is a neutralization reaction?
A reaction between an acid and a base is called a neutralization reaction, and these can be considered to be a type of displacement reaction, where the proton of the acid is being displaced as it is given to another species.
What happens in a neutralization reaction quizlet?
Neutralization occurs when an acid and a base combine. (If you test the pH of the mixture, it would be close to 7, or neutral.) In a neutralization reaction, an acid reacts with a base to produce a salt and water.
How do you write a product of a neutralization reaction?
4.5 Neutralization Reactionsacid + base → water + salt.HCl(aq) + KOH(aq) → H 2O(ℓ) + KCl(aq)2HCl(aq) + Mg(OH) 2(aq) → 2H 2O(ℓ) + MgCl 2(aq)3HCl(aq) + Fe(OH) 3(s) → 3H 2O(ℓ) + FeCl 3(aq)HCl(aq) + NaOH(aq) → H 2O(ℓ) + NaCl(aq)H +(aq) + Cl −(aq) + Na +(aq) + OH −(aq) → H 2O(ℓ) + Na +(aq) + Cl −(aq)More items...
What are the products of a neutralization reaction quizlet?
What are the products of a neutralization reaction? A salt and water.
What is the word equation for the neutralisation reaction?
Example: Writing an Ionic Equation for a Neutralisation Reactionword equation:sulfuric acidpotassium hydroxideskeletal molecular equation:H2SO4(aq)KOH(aq)skeletal ionic equation:2H+(aq) + SO42-(aq)K+ + OH-(aq)
What are the products of a neutralization reaction quizlet?
What are the products of a neutralization reaction? A salt and water.
What two products form acids and bases?
Most introductory chemistry books will teach that the reaction between an acid and a base is called neutralization, and the products formed are water and a salt.
What are the products of the neutralization reaction between HCl and CA OH 2?
Answer and Explanation: When HCl reacts with Ca(OH)2, a salt and water is formed. This is an example of a neutralization reaction.
1. What is the best method to complete a neutralization reaction in a chemistry lab?
Neutralisation reaction is studied in practical classes through titration. An acid solution of a given concentration is added slowly by a burette t...
2. What are the applications of neutralization reactions?
Neutralization reactions have immense application in our day-to-day life. The following are some important applications:It is extremely necessary t...
3. How to handle a bee sting and a wasp sting?
Bee sting or wasp sting both are painful and can be treated instantly using the concept of neutralisation reaction, However the approach for handli...
4. How to know whether a given sample is acid or base?
The following tips can help one to understand whether the given sample is an acid or base.If a drop of the given solution is dropped on a blue litm...
5. What are the different types of salts formed as products of neutralisation reactions?
Mainly three different types of salts are formed from neutralisation reactions based on the strength of the acid and base involved in the neutralis...
When a strong acid neutralizes a strong base, the resultant is neutral?
When a strong acid neutralizes a strong base, the resultant is neutral because all the H+ ions react with all the OH-ions to form water. There are no surplus H+ or OH- ions present in the resultant solution. Its pH is 7.
What is the state when the rate of formation of product is equal to the rate of formation of the reactants?
Weak Acid and Weak Base. Here since both, acid and base, is weak neither of them dissociates completely and so neutralization does not occur. Equilibrium is the state when the rate of formation of product is equal to the rate of formation of the reactants.
What is the pH of a weak base?
Here the amount of OH- ions is very less compared to the amount of H+ ions available. Therefore, the resultant solution is acidic. Its pH lies between 3-6.
What is neutralization reaction?
Neutralization Reaction. Neutralization is a type of chemical reaction in which a strong acid and strong base react with each other to form water and salt. Have you ever been unlucky enough to be stung by a wasp or a bee?
When a strong base neutralizes a weak acid, the pH of the reaction will be greater than 7?
When a strong base neutralizes a weak acid, pH that results from this reaction will be greater than 7 because the strong base dominates over the acid and not all the base in the reaction has been completely neutralized. The same idea goes for when a strong acid neutralizes a weak base.
What happens when a strong acidic solution reacts with a strong basic solution?
When a strong acidic solution reacts with a strong basic solution, neutralization occurs , and drawing from common acids and bases in the table, the reaction will look like the following:
How is salt formed?
A salt is formed when a cation (positive ion) of an base forms a compound with the anion (negative ion) of a acid. The neutralization of a strong acid and a strong base results in a solution with a pH of 7 (neutral pH). The following is a table of common strong acids and strong bases: When a strong acidic solution reacts with a strong basic ...
Why do weak acids retain their molecular form?
So in the following equations, you will see that for weak acids and bases, they retain their molecular form because they do not completely ionize. Here are examples of these reactions and what their net ionic equations will look like:
What is the reaction between a strong acid and a strong base?
Let us take, for example, the reaction of a strong acid and a strong base, such as Hydrogen Bromide (HBr) and Potassium Hydroxide (KOH). The reaction produces water and a soluble salt called Potassium Chloride (KCl). Since the salt is soluble in water, that means it completely dissociates in water and the water remains in its molecular form, ...
When is an electrolyte strong?
We can say that an electrolyte is strong when it ionizes completely in solution. Because of this, we can say that when a strong acidic solution and a strong basic solution both react with each other, they completely dissociate into their respective cations and anions.
Example 12
Oxalic acid, H 2 C 2 O 4 (s), and Ca (OH) 2 (s) react very slowly. What is the net ionic equation between these two substances if the salt formed is insoluble? (The anion in oxalic acid is the oxalate ion, C 2 O 42− .)
Key Takeaways
The Arrhenius definition of an acid is a substance that increases the amount of H + in an aqueous solution.
Exercises
An Arrhenius acid increases the amount of H + ions in an aqueous solution.
What is neutralization in chemical reactions?
Neutralization is a chemical reaction where an acid and a base react with each other quantitatively. It is also written as Neutralization. The acid strength of the reactant gives the pH of the neutralized solution.
What is Neutralization?
It is an acid-base reaction in which an acid reacts with a base to form salt and water. The pH of the neutralized solution depends upon the acid strength of the reactants and their concentrations. The neutralization reaction is best represented as:
What is the reaction of a strong acid to a strong base?
Neutralization Reaction. When a strong acid reacts with a strong base the resultant salt is neither acidic nor basic in nature i.e. it is neutral. For example when HCl ( Hydrochloric acid ), a strong acid, reacts with NaOH, a strong base, then the resulting salt is sodium chloride and water.
What is neutralization in wastewater treatment?
Application of Neutralization. This method is used in wastewater treatment in order to reduce the damage created by the effluents. Neutralization is used in the manufacturing of antacid tablets. The neutralization reaction is used to control the pH of the soil.
What is the result of a strong base and a weak acid?
Likewise when a strong base reacts with a weak acid then the resultant salt is basic in nature. For example, K 2 CO 3 is formed due to the acid-base reaction of potassium hydroxide (strong base) and H 2 CO 3 (weak acid).
When a weak acid and weak base react with each other, complete neutralization does not occur?
When a weak acid and weak base react with each other complete neutralization does not occur due to incomplete ionization of the acid and base.
Is salt a neutral?
Water and salt both are neutral which means, whenever acid and base react together , they are neutralized by each other. Therefore, it is termed as a neutralization reaction. For any further support on acids bases and salts, register with BYJU’S. Test Your Knowledge On Neutralization!
What is neutralization reaction?
Answer. Neutralization reactions are one type of chemical reaction that proceeds even if one reactant is not in the aqueous phase. For example, the chemical reaction between HCl (aq) and Fe (OH) 3 (s) still proceeds according to the equation: even though Fe (OH) 3 is not soluble.
What is the reaction between an acid and a base?
The reaction of an acid and a base is called a. neutralization reaction. . Although acids and bases have their own unique chemistries, the acid and base cancel each other’s chemistry to produce a rather innocuous substance—water. In fact, the general reaction between an acid and a base is. acid + base → water + salt.
What increases the amount of H+ ions in an aqueous solution?
An Arrhenius acid increases the amount of H+ ions in an aqueous solution.
What is salt in chemistry?
(In chemistry, the word salt refers to more than just table salt.) For example, the balanced chemical equation for the reaction between HCl (aq) and KOH (aq) is.
What is an acid in chapter 3?
In Chapter 3 “Atoms, Molecules, and Ions”, in the section called “Acids”, we defined an acid as an ionic compound that contains H + as the cation. This is slightly incorrect, but until additional concepts were developed, a better definition needed to wait. Now we can redefine an acid: an is any compound that increases the amount of hydrogen ion ...
What is the chemical opposite of an acid?
Now we can redefine an acid: an is any compound that increases the amount of hydrogen ion (H +) in an aqueous solution. The chemical opposite of an acid is a base. The equivalent definition of a base is that a is a compound that increases the amount of hydroxide ion (OH −) in an aqueous solution. These original definitions were proposed by ...
Is net ionic equivalent to extra water?
With the exception of the introduction of an extra water molecule, these two net ionic equations are equivalent.
What is neutralization reaction?
Neutralization reactions are one type of chemical reaction that proceeds even if one reactant is not in the aqueous phase. For example, the chemical reaction between HCl (aq) and Fe (OH) 3 (s) still proceeds according to the equation
What is the net ionic reaction?
13. Because the salts are soluble in both cases, the net ionic reaction is just H + (aq) + OH − (aq) → H 2 O (ℓ).
What is salt in chemistry?
(In chemistry, the word salt refers to more than just table salt.) For example, the balanced chemical equation for the reaction between HCl (aq) and KOH (aq) is
What is the difference between an acid and a base?
Now we can redefine an acid: an acid is any compound that increases the amount of hydrogen ion (H +) in an aqueous solution. The chemical opposite of an acid is a base. The equivalent definition of a base is that a base is a compound that increases the amount of hydroxide ion (OH −) in an aqueous solution. These original definitions were proposed by Arrhenius (the same person who proposed ion dissociation) in 1884, so they are referred to as the Arrhenius definition of an acid and a base, respectively.
What increases the amount of H + ions in an aqueous solution?
An Arrhenius acid increases the amount of H + ions in an aqueous solution.
Is net ionic equivalent to extra water?
With the exception of the introduction of an extra water molecule, these two net ionic equations are equivalent.
