What is volatilization gravimetry Quizlet?
Volatilization gravimetry is a mass analysis method that uses thermal or chemical energy to separate substances in order to measure their masses. Which of the following statements best describes the process of volatilization gravimetry? The measurement of the change in concentration after neutralizing a sample
What is an example of gravimetric analysis?
One type of gravimetric analysis is called volatilization gravimetry, which measures the change in mass after removing volatile compounds. An example of volatilization gravimetry would be using the change in mass after heating to calculate the amount or purity of a metal hydrate.
What is indirect volatilization gravimetric analysis?
In an indirect volatilization gravimetric analysis, the change in the sample’s weight is proportional to the amount of analyte. Note that in the following example it is not necessary to apply a conservation of mass to relate the analytical signal to the analyte.
What is the equipment used in volatilization gravimetry?
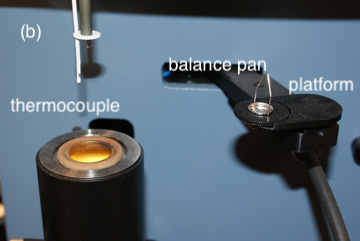
Depending on the method of analysis, the equipment for volatilization gravimetry may be simple or complex. In the simplest experimental design, we place the sample in a crucible and decompose it at a fixed temperature using a Bunsen burner, a Meker burner, a laboratory oven, or a muffle furnace.
What is volatilization method?
The process of converting a chemical substance from a liquid or solid state to a gaseous or vapor state. Other terms used to describe the same process are vaporization, distillation, and sublimation.
Why volatilization gravimetric method is used?
Volatilization gravimetry involves separating out volatile compounds and measuring the change in mass. Using volatilization gravimetry, we can determine the mass of the volatile compound and the mass of the remainder and use these values to calculate the number of moles of each.
What happens during volatilization?
Volatilization is the conversion of a liquid chemical into a vapor, which escapes into the atmosphere.
What are the three types of gravimetric analysis?
Types of Gravimetric Analysis:Precipitation Method: An analytical method called precipitation gravimetry uses a precipitation reaction to separate ions from a solution. ... Volatilization Method: ... Electrogravimetry Method: ... Thermogravimetric Method:
What is the principle of gravimetric analysis?
The principle behind the gravimetric analysis is that the mass of an ion in a pure compound and can be determined. Later, used to find the mass percent of the same ion in a known quantity of an impure compound.
What is application of gravimetric analysis?
Gravimetric analysis is performed with the selective precipitation of the respective component. Chemical analysis of ores and other industrial materials, equipment calibration, and elemental analysis of inorganic compounds are all applications of gravimetric analysis.
What is the significance of volatilization process?
The volatilization process of contaminants has been shown to play an important role in remediation of VOCs-contaminated soils. The aim of this paper is to study the volatilization characteristics of both toluene and water from soil, and to evaluate their interaction mechanism under different conditions.
Why does volatilization happen?
Volatilization is another form of loss of nitrogen (N) as urea. It occurs when surface-spread urea nitrogen is changed to ammonia gas (NH3) and lost into the atmosphere due to a combination of warm weather and the lack of incorporation of the urea into the soil.
What causes volatilization?
What causes ammonia volatilization? The process of ammonia volatilization commonly takes place when nitrogen is in an organic form known as urea. Urea may originate from animal manure, urea fertilizers and, to a lesser degree, the decay of plant materials.
What are two common examples of gravimetric analysis?
The two most common gravimetric methods using volatilization are those for water and carbon dioxide. An example of this method is the isolation of sodium hydrogen bicarbonate (the main ingredient in most antacid tablets) from a mixture of carbonate and bicarbonate.
What are the limitations of gravimetric analysis?
The Disadvantage of Gravimetric Method: The chief disadvantage of this method is that it is very time-consuming. The chemist in today's world prefers other methods over this method. The gravimetric analysis, in general, can provide analysis of a single element, or a limited group of elements, at a time.
Is gravimetric analysis qualitative or quantitative?
quantitative chemical analysisgravimetric analysis, a method of quantitative chemical analysis in which the constituent sought is converted into a substance (of known composition) that can be separated from the sample and weighed.
What are two common examples of gravimetric analysis?
The two most common gravimetric methods using volatilization are those for water and carbon dioxide. An example of this method is the isolation of sodium hydrogen bicarbonate (the main ingredient in most antacid tablets) from a mixture of carbonate and bicarbonate.
Why can't we use tap water in this gravimetric analysis?
Distilled water must be used in the gravimetric analysis of chlorides because in impure water there are other ions which can interfere with the formation of precipitate of chloride or form precipitate along with chlorides which results in errors in calculations of the mass of the chlorine and gravimetric analysis is ...
What is the difference between gravimetric and volumetric analysis?
The gravimetric method establishes the mass of water either contained or delivered from a test measure and hence the associated volume. The volumetric method employs a known volume from which water is transferred to an unknown volume and thereby the contained or delivered volume can be derived.
What are the optimum conditions for precipitation in gravimetric analysis?
All precipitation gravimetric analysis share two important attributes. First, the precipitate must be of low solubility, of high purity, and of known composition if its mass is to accurately reflect the analyte's mass. Second, the precipitate must be easy to separate from the reaction mixture.
How does volatilization gravimetry work?
Volatilization gravimetry involves separating components of our mixture by heating or chemically decomposing the sample. The heating or chemical decomposition separates out any volatile compounds, which results in a change in mass that we can measure. We will go through a detailed example of volatilization gravimetry in the next section of this article!
What is the purpose of gravimetric analysis?
Gravimetric analysis is a class of lab techniques used to determine the mass or concentration of a substance by measuring a change in mass. The chemical we are trying to quantify is sometimes called the analyte. We might use gravimetric analysis to answer questions such as:
What happens when the difference in mass is calculated in step?
In this situation, the difference in mass we calculate in Step will be lower than it should be, so we will have correspondingly fewer moles of water in Step . That will result in calculating a lower percent mass of compared to fully dehydrating the sample. In the end, we will end up underestimating the purity of the metal hydrate.
Can gravimetric analysis be used to determine purity?
We just successfully used gravimetric analysis to calculate the purity of a mixture, hooray! However, sometimes when you are in lab, things might not go quite so smoothly. Things that could go wrong include:
What does volatilization gravimetry mean?
This question is asking us to define volatilization gravimetry. “Volatilization” means that we evaporate or otherwise remove gases with low boiling points. “Gravimetry” means we are measuring a mass. These definitions fit answer C.
What is the purpose of a volatilization gravimetry?
Volatilization gravimetry describes gravimetric analysis methods that use thermal or chemical energy to separate the substances of a mixture or chemical compound. The thermal or chemical energy is used to convert some solid reactant molecules into gaseous molecules.
What is a volatile compound that is separated during volatilization gravimetry?
Volatilization gravimetry is a process that uses chemical or thermal energy to separate volatile compounds. A volatile compound is a compound with a low boiling point.
What is a volatile compound?
A volatile compound is a compound with a low boiling point. During volatilization gravimetry, volatile compounds can easily separate as a gas. In this reaction, the gaseous product that emerges is carbon dioxide ( C O 2 ). We can use these statements to determine that option A is the correct answer for this question.
What is the water of crystallization?
Water of crystallization is the presence of water molecules within the structure of a crystal.
Is neutralization directly relevant to volatilization gravimetry?
Neutralization is not directly relevant to volatilization gravimetry, so answer A is incorrect. We are concerned with the change in mass, not the change in volume, so choice B is incorrect. Choice D describes another type of gravimetry, precipitation gravimetry.
What is volatilization in science?
What is Volatilization? The volatilization Is the process of converting a chemical substance from a liquid or solid state to a gaseous or vapor state. Other terms used to describe the same process are vaporization , distillation Y sublimation . One substance can often be separated from another by volatilization and can then be recovered by ...
What is the purpose of gravimetric analysis?
Gravimetric analysis is a class of laboratory techniques used to determine the mass or concentration of a substance by measuring a change in mass. The chemical we are trying to quantify is sometimes called the analyte. We could use gravimetric analysis to answer questions such as: What is the concentration of analyte in ...
How does the presence of solute affect the vapor pressure?
The presence of solute decreases the number of solvent molecules at the interface, reducing the vapor pressure.
What is vapor pressure?
The vapor pressure is a measure of the tendency of a material to change to the gaseous or vapor state, ie a measure of the volatility of the substances. As the vapor pressure increases, the greater the ability of the liquid or the solid to evaporate thereby becoming more volatile. The vapor pressure will increase with temperature.
What is the property of vapor pressure?
The responsible for all this is a property known as vapor pressure, which is the pressure exerted by a vapor in equilibrium with the solid or liquid phase of the same substance. Also, the partial pressure of the substance in the atmosphere on the solid or liquid (Anne Marie Helmenstine, 2014). The vapor pressure is a measure of the tendency ...
What is the role of the difference in volatilities of compounds in a mixture?
The difference in volatilities of the compounds of the mixture plays a fundamental role in their separation.
Can we trap and weigh a volatile product of decomposition?
Alternatively, we can trap and weigh a volatile product of decomposition. Because releasing a volatile species is an essential part of these methods, we classify them collectively as gravimetric methods of volatilization (Harvey, 2016).
What is gravity analysis?
Gravimetric analysis is a type of lab technique used to determine the mass or concentration of a substance by measuring a change in mass. The chemical we are trying to quantify is also known as the analyte. In other words, it is a technique through which the amount of an analyte (the ion being analyzed) can be determined through the measurement ...
What is physical gravimetry?
Physical gravimetry is one of the most common types used in environmental engineering. Of these methods, there are 2 common types in volving changes in the phase of the analyte to separate it from the rest of a mixture, resulting in a change in mass.
How to determine particulate mass concentration?
Gravimetric analyses depend on comparing the masses of two compounds which contain the analyte. It is thought to be the most accurate method of determining particulate mass concentration, as they are capable of sampling at the very lowest detection limits. A model is taken by drawing a calculated volume of air through a collection substrate, which is sent for further analysis. Gravimetric analysis is acclaimed as the most accurate method because it is the process of producing and weighing a compound or element in as pure form as possible after any type of chemical treatment has been carried out on the substances which have to be examined.
Which method is the most accurate for determining particulate mass concentration?
Gravimetric analyses depend on comparing the masses of two compounds which contain the analyte. It is thought to be the most accurate method of determining particulate mass concentration, as they are capable of sampling at the very lowest detection limits.
Does gravity require a series of standards?
Gravimetry provides only very little room for instrumental error and it also does not require a series of standards for calculating the unknown.