What are the uses of xanthine derivatives?
The major use of xanthine derivatives are for relief of bronchospasm caused by asthma or chronic obstructive lung disease. The most widely used xanthine is theophylline.
What is the oxidation of xanthine?
Xanthine is an intermediate in the degradation of adenosine monophosphate to uric acid, being formed by oxidation of hypoxanthine. The methylated xanthine compounds caffeine, theobromine, and theophylline and their derivatives are used in medicine for their bronchodilator effects.
What are the side effects of xanthine?
GENERIC NAME: XANTHINE DERIVATIVES - ORAL. SIDE EFFECTS: Dizziness, headache, lightheadedness, heartburn, stomach pain, loss of appetite, restlessness, nervousness, sleeplessness or increased urination may occur as your body adjusts to the medication. If these symptoms persist or worsen, inform your doctor.
What are xanthines and caffeine?
Xanthines also include medicines such as theophylline, used in the treatment of asthma. Caffeine is a naturally occurring substance which stimulates the central nervous system and can temporarily relieve tiredness and increase alertness 15).
What do xanthine derivatives do?
Xanthine derivatives are a group of alkaloids that work as mild stimulants and bronchodilators. Xanthine derivatives ease symptoms of bronchospasm and make breathing easier by relaxing the smooth muscles of the respiratory tract and reducing the airway's hypersensitive response to stimuli.
What is an example of xanthine derivative?
OVERVIEW. The xanthine derivatives are agents that resemble natural occurring xanthines such as caffeine, theobromine and methylxanthines. These are plant alkaloids and components of coffee, tea and chocolate.
Which is the most commonly used xanthine derivative?
Caffeine is the most important xanthine alkaloid. It is a mildly stimulant drug found in tea, coffee, cocoa, and the kola nut and is usually associated with the alkaloids theophylline and theobromine, which are mild cardiac stimulants.
What are theophylline and xanthine derivatives?
Theophylline is a xanthine derivative. Theophylline relaxes bronchial smooth muscle by inhibition of the enzyme phosphodiesterase and suppresses airway responsiveness to stimuli that cause bronchoconstriction.
What are the three xanthines?
Occurrence. The xanthine alkaloids include caffeine, theobromine, and theophylline, and are well-known components of tea (Camellia sinensis), coffee (Coffea arabica), cola ingredients (Cola spp.), and cocoa (Theobroma cacao).
Where is xanthine found?
Xanthine: A substance found in caffeine, theobromine, and theophylline and encountered in tea, coffee, and the colas.
Is coffee a xanthine?
Caffeine, theobromine, and theophylline (Figure 1) belong to a group of compounds known as the xanthines. Caffeine (1,3,7-trimethylxanthine) and theobromine (3,7- dimethylxanthine) (1) are in such beverages as coffee, teas and colas and are common to the urine of these drinkers (2).
What are some natural sources for xanthines?
The natural source of xanthine and its derivatives are plants as tea, coffee, cocoa seeds, etc. The presence of natural xanthine derivatives in plant is good for human health but their precise biological role in plants still needs exploration [36].
What is the mechanism of action for xanthines?
Action & molecular mechanism The main mechanism of action of xanthine is represented by the inhibition of phosphodiesterase, enzyme that breaks a phosphodiester bond. The pharmacological activity of xanthine is expressed in smooth muscle, heart muscle, central nervous system and kidney.
What is xanthene derivatives?
The xanthene derivatives methantheline (Banthine) (728; R=Et) and propantheline (Pro-Banthine) (728; R=Pri) are effective compounds for the treatment of ulcers and gastrointestinal disorders, reducing the volume and acidity of gastric secretions.
What are the three types of bronchodilators?
For treating asthma symptoms, there are three types of bronchodilators: beta-agonists, anticholinergics, and theophylline. You can get these bronchodilators as tablets, liquids, and shots, but the preferred way to take beta-agonists and anticholinergics is inhaling them.
What kind of drug is theophylline?
Theophylline belongs to a group of medicines known as bronchodilators. Bronchodilators are medicines that relax the muscles in the bronchial tubes (air passages) of the lungs. They relieve cough, wheezing, shortness of breath, and troubled breathing by increasing the flow of air through the bronchial tubes.
What is xanthene derivatives?
The xanthene derivatives methantheline (Banthine) (728; R=Et) and propantheline (Pro-Banthine) (728; R=Pri) are effective compounds for the treatment of ulcers and gastrointestinal disorders, reducing the volume and acidity of gastric secretions.
Is coffee a xanthine?
Caffeine, theobromine, and theophylline (Figure 1) belong to a group of compounds known as the xanthines. Caffeine (1,3,7-trimethylxanthine) and theobromine (3,7- dimethylxanthine) (1) are in such beverages as coffee, teas and colas and are common to the urine of these drinkers (2).
What are some natural sources for xanthines?
The natural source of xanthine and its derivatives are plants as tea, coffee, cocoa seeds, etc. The presence of natural xanthine derivatives in plant is good for human health but their precise biological role in plants still needs exploration [36].
What is a adverse effect of xanthine derivatives?
SIDE EFFECTS: Dizziness, headache, lightheadedness, heartburn, stomach pain, loss of appetite, restlessness, nervousness, sleeplessness or increased urination may occur as your body adjusts to the medication. If these symptoms persist or worsen, inform your doctor.
What is Xanthine derivative?
Xanthine derivatives are medications used to treat bronchospasm caused by lung conditions such as asthma. Xanthine is a naturally occurring compound in the human body and is also found in plant products such as tea, coffee, and cocoa beans.
Does xanthine increase cAMP?
Xanthine derivatives increase the cellular levels of signaling molecules known as cyclic adenosine monophosphate (cAMP) by inhibiting the activity of phosphodiesterase, an enzyme that regulates cAMP levels. An increase in tissue concentration of cAMP results in bronchial smooth muscle relaxation and bronchodilation.
What class is Xanthine derivative?
Xanthine derivatives have been given a class IIb (level of evidence B) by the ESC for the treatment of patients with microvascular angina.
What is the purpose of xanthines?
Owing to their ability to relax bronchial muscles, the xanthines are used chiefly in the maintenance treatment of asthma and chronic obstructive pulmonary disease.
How many names end in phylline?
The WHO list of International Nonproprietary Names (INNs) includes 46 names ending in –fylline or -phylline (albifylline, aminophylline, apaxifylline, arofylline, bamifylline, cipamfylline, denbufylline, derenofylline, dimabefylline, diniprofylline, diprophylline, doxofylline, enprofylline, etamiphylline, etofylline, flufylline, fluprofylline, furafylline, guaifylline, isbufylline, istradefylline, laprafylline, lisofylline, lomifylline, mercurophylline, metescufylline, mexafylline, midaxifylline, naxifylline, nestifylline, pentifylline, pentoxifylline, perbufylline, pimefylline, propentofylline, proxyphylline, pyridofylline, rolofylline, spirofylline, stacofylline, tazifylline, tonapofylline, torbafylline, triclofylline, verofylline, visnafylline). Not all have been used to treat asthma. For example, the adenosine A 2A receptor antagonist istradefylline has been used to treat Parkinson’s disease [ 1, 2 ]; pentoxifylline (which is partly converted in vivo to lisofylline and is covered in a separate monograph) has been used to treat a range of conditions, such as leg ischemia exercise in patients with intermittent claudication and to suppress overproduction of tumor necrosis factor alfa in conditions such as falciparum malaria and rheumatoid arthritis and in transplant recipients, with varied success; lisofylline (which is converted in vivo to pentoxifylline) has been used to treat acute lung damage and acute respiratory distress syndrome and diabetes mellitus; pentifylline for acute ischemic stroke and hyperlipoproteinemia; propentofylline for acute ischemic stroke and dementias; and rolofylline and tonapofylline for acute heart failure.
Where is Xanthine found?
All three are present in maté ( Ilex paraguarensis) leaves, 400,401 and theobromine ( 181 )/caffeine ( 183) (and ∼300 other natural products) accumulate in cocoa beans from cacao ( Theobroma cacao ). 440 Caffeine ( 183) is also part of the extracts from tea ( Camellia sinensis) leaves and coffee ( Coffea arabica) beans (see Chapters 3.21 and 3.22 ).
What is the ratio of aminophylline to ethylenediamine?
Aminophylline. A mixture of theophylline with ethylenediamine in a ratio of about 85:15, in order to increase solubility of the xanthine; the ethylenediamine may cause hypersensitivity reactions; see monograph on Theophylline. Bamifylline.
What is XOXs in biology?
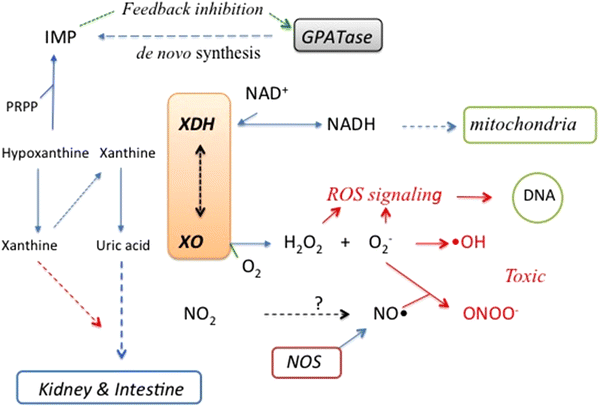
Xanthine oxidases (XOxs) are a class of enzymes that generate reactive oxygen species. These enzymes catalyze the oxidation of hypoxanthine and can further catalyze the oxidation of xanthine to uric acid. These enzymes play an important role in the catabolism of purines in humans.
How much sulfate is in a day?
4.4–6.6 mg/kg/day in divided doses.
What class is Xanthine derivative?
Xanthine derivatives have been given a class IIb (level of evidence B) by the ESC for the treatment of patients with microvascular angina.
What are the Xanthine alkaloids?
The xanthine alkaloids include caffeine, theobromine, and theophylline, and are well-known components of tea ( Camellia sinensis ), coffee ( Coffea arabica ), cola ingredients ( Cola spp.), and cocoa ( Theobroma cacao ). These are obviously consumed in considerable quantity around the world. Caffeine is the most important xanthine alkaloid. It is a mildly stimulant drug found in tea, coffee, cocoa, and the kola nut and is usually associated with the alkaloids theophylline and theobromine, which are mild cardiac stimulants. Coffee seeds (beans) show considerable variations in caffeine concentration, as do the various brews prepared from the roasted and ground bean. A typical serving of coffee contains between 40 and 100 mg of caffeine. Coffee contains trace amounts of theophylline, but no theobromine. The tea plant contains more caffeine than coffee, however, the brewing process results in the caffeine content of the beverage being rather lower than that of coffee.
How many names end in phylline?
The WHO list of International Nonproprietary Names (INNs) includes 46 names ending in –fylline or -phylline (albifylline, aminophylline, apaxifylline, arofylline, bamifylline, cipamfylline, denbufylline, derenofylline, dimabefylline, diniprofylline, diprophylline, doxofylline, enprofylline, etamiphylline, etofylline, flufylline, fluprofylline, furafylline, guaifylline, isbufylline, istradefylline, laprafylline, lisofylline, lomifylline, mercurophylline, metescufylline, mexafylline, midaxifylline, naxifylline, nestifylline, pentifylline, pentoxifylline, perbufylline, pimefylline, propentofylline, proxyphylline, pyridofylline, rolofylline, spirofylline, stacofylline, tazifylline, tonapofylline, torbafylline, triclofylline, verofylline, visnafylline). Not all have been used to treat asthma. For example, the adenosine A 2A receptor antagonist istradefylline has been used to treat Parkinson’s disease [ 1, 2 ]; pentoxifylline (which is partly converted in vivo to lisofylline and is covered in a separate monograph) has been used to treat a range of conditions, such as leg ischemia exercise in patients with intermittent claudication and to suppress overproduction of tumor necrosis factor alfa in conditions such as falciparum malaria and rheumatoid arthritis and in transplant recipients, with varied success; lisofylline (which is converted in vivo to pentoxifylline) has been used to treat acute lung damage and acute respiratory distress syndrome and diabetes mellitus; pentifylline for acute ischemic stroke and hyperlipoproteinemia; propentofylline for acute ischemic stroke and dementias; and rolofylline and tonapofylline for acute heart failure.
Where is Xanthine found?
All three are present in maté ( Ilex paraguarensis) leaves, 400,401 and theobromine ( 181 )/caffeine ( 183) (and ∼300 other natural products) accumulate in cocoa beans from cacao ( Theobroma cacao ). 440 Caffeine ( 183) is also part of the extracts from tea ( Camellia sinensis) leaves and coffee ( Coffea arabica) beans (see Chapters 3.21 and 3.22 ).
What is xanthine used for?
Owing to their ability to relax bronchial muscles, the xanthines are used chiefly in the maintenance treatment of asthma and chronic obstructive pulmonary disease.
How long does caffeine last?
Taken orally, caffeine is rapidly absorbed. The half-life of caffeine is 3.5 to 5 hours. The behavioral effects of caffeine include increased mental alertness, a faster and clearer flow of thought, wakefulness, and restlessness. 61 Fatigue is reduced and sleep-onset delayed. 61 The physical effects of caffeine include palpitations, hypertension, increased gastric acid secretion, and increased urine output. 61 Heavy consumption (12 or more cups/day, or 1.5 g of caffeine) causes agitation, anxiety, tremors, rapid breading, and insomnia. 61
What is the ratio of aminophylline to ethylenediamine?
Aminophylline. A mixture of theophylline with ethylenediamine in a ratio of about 85:15, in order to increase solubility of the xanthine; the ethylenediamine may cause hypersensitivity reactions; see monograph on Theophylline. Bamifylline.
What are the Xanthine medications?
The xanthines, caffeine and theobromine are the pharmacologically active components of a range of drinks such as coffee, tea, cocoa and soft drinks. Xanthines also include medicines such as theophylline, used in the treatment of asthma.
What is xanthine made of?
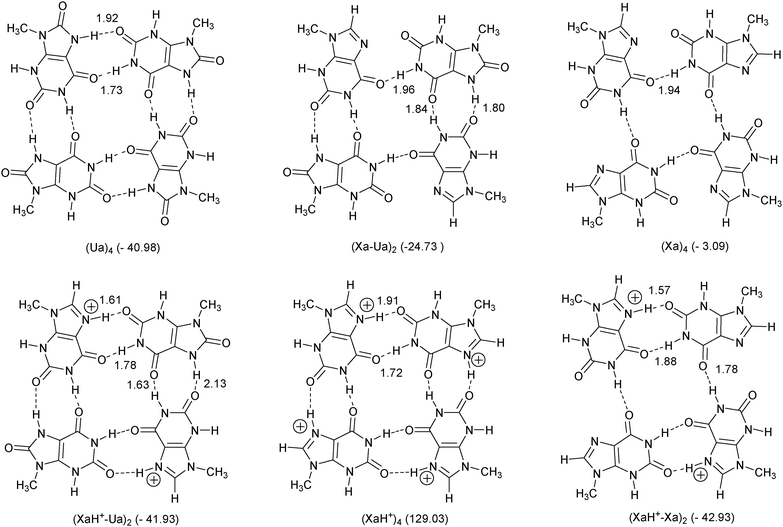
Xanthine is formed following enzymatic degradation of adenine and guanine. The term “xanthines” denotes a wide class of compounds whose central core (3,7-dihydro-purine-2,6-dione) is closely related to the DNA bases guanine and adenine (see Figures 1 and 2) 1). Like nucleobases, xanthines are capable of forming hydrogen bonds, allowing their insertion in duplexes 2) and guanine quadruplexes (G-quadruplexes) 3). Moreover, it has been shown that their self-association can give rise to four-stranded structures 4), which have attracted attention for applications in the field of molecular electronics 5). Moreover, methylxanthines are used as therapeutic agents acting, among others, as stimulants of the nervous system 6). The well-known caffeine, present in coffee and tea and various soft beverages, is none other than 1,3,7-trimethylxanthine (Figure 2). It is also worth noticing that 2′-deoxyxanthosine (dX) has been used in the extension of the genetic alphabet by purine pairing with a 2,4-diaminopyrimidine nucleoside, which has a hydrogen bonding pattern complementary to 2′-deoxyxanthosine (dX) 7).
What is the best medicine for gout?
Xanthine oxidase inhibitor. Allopurinol and Febuxostat are xanthine oxidase inhibitor drugs that is used to treat gout and high levels of uric acid in the blood (hyperuricemia) caused by certain cancer medications, and kidney stones. Xanthine oxidase inhibitor works by causing less uric acid to be produced by the body.
What gene prevents xanthine dehydrogenase from turning on?
Mutations in the MOCOS gene prevent xanthine dehydrogenase and aldehyde oxidase from being turned on (activated). The loss of xanthine dehydrogenase activity prevents the conversion of xanthine to uric acid, leading to an accumulation of xanthine in the kidneys and other tissues.
What is xanthinuria in the kidneys?
Xanthine oxidase deficiency. Hereditary xanthinuria is a condition that most often affects the kidneys. It is characterized by high levels of a compound called xanthine and very low levels of another compound called uric acid in the blood and urine. The excess xanthine can accumulate in the kidneys and other tissues.
Does xanthine cause uric acid?
Because xanthine is not converted to uric acid, affected individuals have high levels of xanthine in their blood (hyperxanthinemia) and urine (xanthinuria) and very low levels of uric acid in their blood and urine. The excess xanthine can cause damage to the kidneys and other tissues.
Is xanthine a curative treatment?
There is no curative treatment for hereditary xanthinuria. The only recommended treatment for patients with xanthinuria is a low purine diet and high intake of fluids 11). Because the solubility of xanthine is relatively independent of urinary pH, urine alkalinization has no effect (in contrast to patients with uric acid lithiasis) 12). When kidney stones are present, a pyelolithotomy might be necessary.
a. Caffeine
Properties and uses: It exists as a white crystalline powder or silky white crystals, sublimes readily, sparingly soluble in water, freely soluble in boiling water, and slightly soluble in ethanol. It dissolves in the concentrated solutions of alkali benzoates or salicylates.
b. Theophylline and Theobromine (Theobid, Theopa, Broncordil)
Synthesis: They can be synthesized by adopting the above route described for caffeine with the suitable reagents.
i. Theophylline hydrate
Properties and uses: It exits as white, crystalline powder, slightly soluble in water, sparingly soluble in ethanol. It dissolves in solutions of alkali hydroxides, in ammonia, and in mineral acids. Used as nonselective phosphodiesterase inhibitor (xanthine) and in the treatment of reversible airways obstruction.
ii. Theobromine
Properties and use: It is a white powder, very slightly soluble in water and in ethanol, slightly soluble in ammonia. It dissolves in dilute solutions of alkali hydroxides and in mineral acids. Theobromine is used as diuretic and used in the treatment of angina pectoris and hypertension.
c. Aminophylline (Aminophyline, Minophyl)
Properties and uses: A white or slightly yellowish powder, sometimes granular, freely soluble in water (the solution becomes cloudy through absorption of carbon dioxide), practically insoluble in ethanol.
GENERIC NAME: XANTHINE DERIVATIVES - ORAL
USES: This medication improves breathing by opening air passages in the lungs. It is used in the treatment of asthma, chronic bronchitis, and emphysema. This medication works best when taken on an empty stomach one hour before or two hours after meals. If stomach upset occurs, it may be taken with food.
Report Problems to the Food and Drug Administration
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit the FDA MedWatch website or call 1-800-FDA-1088.
