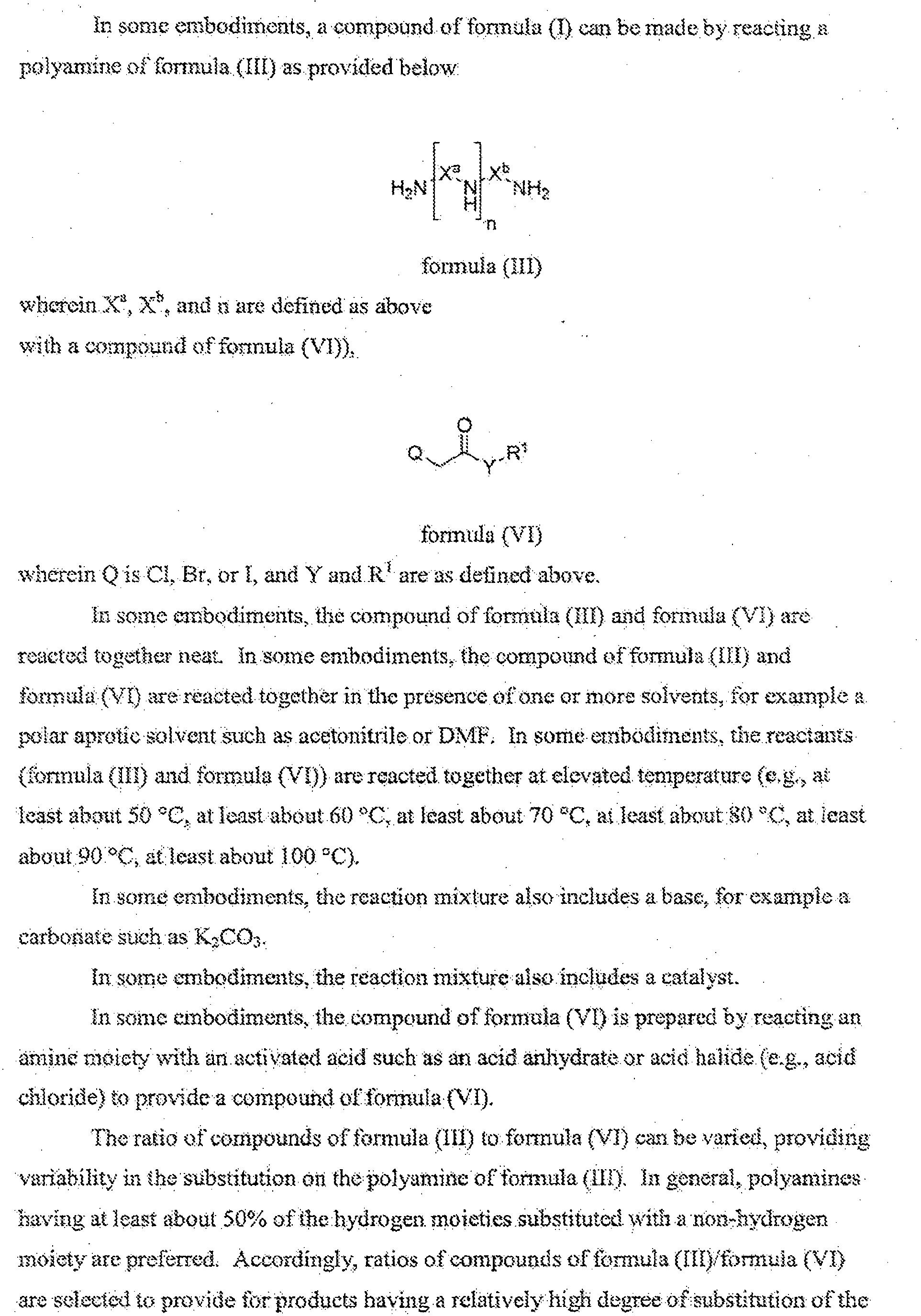
The simplest definition is a lipid as any molecule that is insoluble in water and soluble in organic solvents. Most lipids
Lipid
In biology and biochemistry, a lipid is a biomolecule that is soluble in nonpolar solvents. Non-polar solvents are typically hydrocarbons used to dissolve other naturally occurring hydrocarbon lipid molecules that do not dissolve in water, including fatty acids, waxes, sterols, fat-soluble vitamins, monoglycerides, diglycerides, triglycerides, and phospholipids.
Benzene
Benzene is an organic chemical compound with the chemical formula C₆H₆. The benzene molecule is composed of six carbon atoms joined in a ring with one hydrogen atom attached to each. As it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon.
What substances are lipids soluble in?
Lipids are non-polar organic compounds. Hence they are soluble in organic solvents such as ethanol (alcohol), but insoluble in water. Ethanol is an organic substance and so dissolves other organic substances; it is frequently used as an organic solvent. Ethanol extracts the lipid from the crushed solid sample.
What is the general formula of lipids?
What is the general form of lipids? Lipids consist of repeating units called fatty acids. Fatty acids are organic compounds that have the general formula CH3(CH2)nCOOH, where nusually ranges from 2 to 28 and is always an even number. There are two types of fatty acids: saturated fatty acids and unsaturated fatty acids.
What are some common lipids?
Some common examples of lipids are fats, waxes, soluble vitamins (A,D,E &K), sterols, monoglycerides, diglycerides, triglycerides, phospholipids, etc. The representative images of some of the biologically available lipids are as follows.
What are the three groups of lipids?
The four main groups of lipids include:
- Fatty acids (saturated and unsaturated)
- Glycerides (glycerol-containing lipids)
- Nonglyceride lipids (sphingolipids, steroids, waxes)
- Complex lipids (lipoproteins, glycolipids)

What solvents are lipids soluble?
AcetoneBenzeneChloroformTolueneLipid/Soluble in
Is lipid soluble in nonpolar solvent?
Lipids Are Defined by Solubility and Intermolecular Forces As alluded to above, lipids are a class of naturally occurring molecules that are soluble in nonpolar organic solvents, and are not soluble in water.
Are lipids soluble in organic solvents?
In general, neutral lipids are soluble in organic solvents and are not soluble in water. Some lipid compounds, however, contain polar groups which, along with the hydrophobic part, impart an amphiphilic character to the molecule, thus favoring the formation of micelles from these compounds.
Is lipid a polar solvent?
Water molecules are polar because they have positive and negative ends, rather like little magnets. Most lipids are non-polar (having no charged areas) or only slightly polar, with a very few charged areas. Water mixes with hydrophilic (water-loving) compounds by sticking to their charged groups.
What are the non-polar solvents?
Nonpolar solvents include alkanes (pentane, hexane, and heptane) and aromatics (benzene, toluene, and xylene). Other common nonpolar solvents include acetic acid, chloroform, diethyl ether, ethyl acetate, methylene chloride, and pyridine.
Why lipids are non-polar?
Lipids include a diverse group of compounds that are largely nonpolar in nature. This is because they are hydrocarbons that include mostly nonpolar carbon–carbon or carbon–hydrogen bonds. Non-polar molecules are hydrophobic (“water fearing”), or insoluble in water. Lipids perform many different functions in a cell.
Why are lipids soluble in nonpolar organic solvents?
They have long chains of nonpolar bonds, which makes them easily dissolvable in oil and grease; but they also have a polar charged group at one end, which makes them easily dissolvable in water.
Why are lipids soluble in ethanol?
The solubility of these lipids increase in alcoholic solvents as the carbon chain length of the alcohol increases, so they are more soluble in ethanol and n-butanol. The shorter chain fatty acids in the lipids will have greater solubility in the more polar solvents.
What is classified as an organic solvent?
Organic solvents are carbon-based substances capable of dissolving or dispersing one or more other substances. Organic solvents can be carcinogens, reproductive hazards, and neurotoxins. Carcinogenic organic solvents include benzene, carbon tetrachloride, and trichloroethylene.
What are lipids soluble in?
AcetoneBenzeneChloroformTolueneLipid/Soluble in
What is the best solvent for lipids?
Lipids are all insoluble in polar solvents like water but highly soluble in the non-polar or weakly polar organic solvents, including ether, chloroform, benzene, and acetone. In fact, these four solvents are often referred to as "lipid-solvents" or "fat-solvents".
Are lipids soluble in acid?
No worries! We've got your back. Try BYJU'S free classes today!
Why are lipids soluble in nonpolar solvents?
They have long chains of nonpolar bonds, which makes them easily dissolvable in oil and grease; but they also have a polar charged group at one end, which makes them easily dissolvable in water.
What are lipids soluble in?
AcetoneBenzeneChloroformTolueneLipid/Soluble in
Which solvent has the greatest lipid solubility?
What is the best solvent to dissolve the lipid? The solvents most used for delivery of lipids to biological systems are ethanol and dimethylsulfoxide (DMSO).
Is lipids are soluble in water True or false?
Lipids are not soluble in water as they are non-polar, but are thus soluble in non-polar solvents such as chloroform.