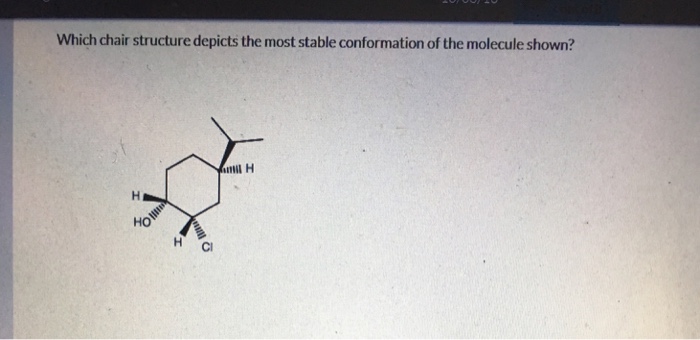
What makes a chair conformation stable? The reason is that when substituents are in the axial position, there tends to be more unfavorable interactions with other axial atoms on the same side. When substituents are in the equatorial position, they are farther away from each other. This increases the stability of the conformation.
How do you determine the stability of chair conformations?
When drawing chair conformations, the type of bond alternates. If carbon one is axial, then carbon two is equatorial. Some chair conformations are more stable than the others and therefore preferred. A key factor for determining stability is to calculate the 1,3-diaxial strain.
Why are some chair conformations preferred over others?
Some chair conformations are more stable than the others and therefore preferred. A key factor for determining stability is to calculate the 1,3-diaxial strain. This is a type of steric interaction that occurs when bulky attachments are located on axial bonds.
How to choose the more stable chair conformation of cyclohexane?
So, choosing the more stable chair conformation is straightforward when there is only one group on the cyclohexane. You just need to find the energy value for the axial group: However, if there are more groups on the cyclohexane, we need to take into consideration the 1,3-diaxial interaction of all.
What type of bonds are used in chair conformations?
This means if you have a methyl on carbon 4, you should place that same methyl group on the carbon labeled 4. Carbon-carbon bonds that are a part of the cyclohexane are drawn as part of the chair’s basic structure. Apart from these are two types of bonds we use in chair conformations: axial and equatorial.

How do you know which chair conformation is the most stable?
To Determine Chair Conformation Stability, Add Up The A-Values For Each Axial Substituent. The Lower The Number, The More Stable It is.
What makes a chair conformation the most stable group of answer choices?
In the previous section, it was stated that the chair conformation in which the methyl group is equatorial is more stable because it minimizes steric repulsion, and thus the equilibrium favors the more stable conformer. This is true for all monosubstituted cyclohexanes.
What makes a cyclohexane stable?
Cyclohexane is the most stable cycloalkane. It is strain-free, meaning neither angle strains nor torsional strains apply, and it shows the same stability as chain alkanes. This special stability is due to a unique conformation that it adopts.
In which conformation has more stability?
Learn about this topic in these articles: …with respect to the other—the eclipsed conformation is the least stable, and the staggered conformation is the most stable. The eclipsed conformation is said to suffer torsional strain because of repulsive forces between electron pairs in the C―H bonds of adjacent carbons.
What is the most stable conformer?
The most stable conformation is anti at both bonds, whereas less stable conformations contain gauche interactions. One gauche-gauche conformer is particularly unfavorable because methyl groups are aligned with parallel bonds in close proximity. This conformation is called syn.
Which conformation is more stable boat or chair?
The chair conformation is more stable than the boat conformation. The boat conformation can sometimes be more stable than it is usually, by a slight rotation in the C-C bonds and is called the skew boat conformation. Nevertheless, the chair conformation is the most stable cyclohexane form.
Which conformation of cyclohexane has highest stability?
The chair conformationThe chair conformation is the most stable conformer. At 298 K (25 °C), 99.99% of all molecules in a cyclohexane solution adopt this conformation.
Is equatorial or axial more stable?
For a 50:50 mixture (K = 1) the energy difference ΔG would be zero. For methylcyclohexane at room temperature (298 K) the 95:5 ratio of equatorial to axial conformers translates to an energy difference of 1.70 kcal/mol. In other words, the equatorial conformer is more stable by 1.70 kcal/mol.
Why is half chair conformation least stable?
The half chair, formed by raising the footrest of the chair, has five of the six C atoms in a plane and one C atom out of the plane. Therefore, it has both eclipsing and bond angle strains and hence is the least stable conformation of cyclohexane.
Which conformation is stable and why?
Staggered conformation of ethane is most stable while eclipsed conformation is least stable because staggered form has the least torsional strain and the eclipsed form has the maximum torsional strain.
Which of these conformations is more stable and why?
Which of these conformations is more stable and why? Answer: The staggered conformation is most stable because the hydrogens and bonding pairs of electrons are at maximum distance. This causes minimum repulsion.
Which conformation is least stable?
The least stable conformation of cyclohexane is half chair conformation.
Core Concepts
In this tutorial brought to you by the ChemTalk team, you will learn about chair conformations. More specifically, you will learn how to convert 2D cyclohexane structures into 3D chair conformations, determine the stability of the conformation, and complete a chair flip.
What are chair conformations?
Chair conformations are the most stable conformations cyclohexane can form. The basic structure is shown below. Each point represents carbon. To help visualize, it should be noted that carbon 1 is pointing up above the plane and carbon 4 is pointing down below the plane. Every other carbon lies in the same plane.
Stability and Ring Flips
Some chair conformations are more stable than the others and therefore preferred. A key factor for determining stability is to calculate the 1,3-diaxial strain. This is a type of steric interaction that occurs when bulky attachments are located on axial bonds.
Organic Chemistry
In the previous two posts, we have talked about drawing the ring-flip of chair conformations and the A value (1,3-diaxial interactions). And we learned that for a given cyclohexane, the axial conformer is less stable than the corresponding equatorial conformer.
Alkanes and Cycloalkanes
In the previous two posts, we have talked about drawing the ring-flip of chair conformations and the A value (1,3-diaxial interactions). And we learned that for a given cyclohexane, the axial conformer is less stable than the corresponding equatorial conformer.

CORE Concepts
Covered in Other Articles
- Newman Projections
- Fischer Projections
- Naming Cycloalkanes
What Are Chair Conformations?
- Chair conformations are the most stable conformations cyclohexane can form. The basic structure is shown below. Each point represents carbon. To help visualize, it should be noted that carbon 1 is pointing up above the plane and carbon 4 is pointing down below the plane. Every other carbon lies in the same plane. Moreover, the conformation continuously jumps back and f…
Stability and Ring Flips
- Some chair conformations are more stable than the others and therefore preferred. A key factor for determining stability is to calculate the 1,3-diaxial strain. This is a type of steric interactionthat occurs when bulky attachments are located on axial bonds. To calculate the 1,3-diaxial strain, you must add the strain energies of substituents loca...
Further Reading