
- The first reaction of pyrimidine synthesis is the synthesis of carbamoyl phosphate by utilizing the amide form of glutamine (glutamate) and HCO3- (carbonic acid).
- This reaction is catalyzed by carbamoyl phosphate synthetase-II. The enzyme is a cytosolic enzyme.
- In this reaction, two ATP molecules are consumed.
What is pyrimidine and an example of it?
The pyrimidine ring system has wide occurrence in nature as substituted and ring fused compounds and derivatives, including the nucleotides cytosine, thymine and uracil, thiamine (vitamin B1) and alloxan. It is also found in many synthetic compounds such as barbiturates and the HIV drug, zidovudine.
What does the term pyrimidine mean?
Pyrimidine Definition. Pyrimidines are simple aromatic compounds composed of carbon and nitrogen atoms in a six-membered ring. The term pyrimidine is also used to refer to pyrimidine derivatives, most notably the three nitrogenous bases that, along with the two purines, are the building blocks of both deoxyribonucleic acid (DNA) and ribonucleic acid (RNA).
Which nucleotides are purines and which are pyrimidine?
Purines and Pyrimidines are nitrogenous bases that make up the two different kinds of nucleotide bases in DNA and RNA.The two-carbon nitrogen ring bases (adenine and guanine) are purines, while the one-carbon nitrogen ring bases (thymine and cytosine) are pyrimidines.
What does pyrimidines mean?
pyrimidine (Noun) A diazine in which the two nitrogen atoms are in the meta- positions; it is the basis of three of the bases found in DNA and RNA, thymine, uracil and cytosine

What makes up a pyrimidine?
Pyrimidine: A nitrogenous base similar to benzene (a six-membered ring) and includes cytosine, thymine, and uracil as bases used for DNA or RNA.
What bases are pyrimidines and what makes them a pyrimidine?
Pyrimidine structure contains a carbon-nitrogen ring which can be modified to form a variety of derivatives. Pyrimidines form the nucleotides, or bases, cytosine, thymine, and uracil found in DNA and RNA and are thus essential for establishing our genetic code.
What is a pyrimidine in simple terms?
Listen to pronunciation. (py-RIH-mih-deen) One of two chemical compounds that cells use to make the building blocks of DNA and RNA. Examples of pyrimidines are cytosine, thymine, and uracil.
Which molecules are pyrimidines?
Uracil, cytosine, and thymine are the principal pyrimidines which constitute uridine, cytidine, and thymidine ribonucleosides and the corresponding deoxynucleosides. Cytosine and thymine are the building blocks of DNA, while cytosine and uracil are found in RNA.
What bases are considered pyrimidines?
The pyrimidine bases are thymine (5-methyl-2,4-dioxipyrimidine), cytosine (2-oxo-4-aminopyrimidine), and uracil (2,4-dioxoypyrimidine) (Fig. 6.2).
What makes a purine different from a pyrimidine?
They are nitrogenous bases that make up the two different nucleotides in DNA and RNA. Purines (adenine and guanine) are two-carbon nitrogen ring bases while pyrimidines (cytosine and thymine) are one-carbon nitrogen ring bases.
What is pyrimidine nucleotide?
In pyrimidine nucleotides, the nitrogenous base is a pyrimidine ring, while purine nucleotides contain a purine ring (a pyrimidine ring joined with an imidazole ring). 2,3. Adenine and guanine are purine nucleotides, while cytosine, uracil, and thymine are pyrimidine nucleotides.
Why is uracil a pyrimidine?
Uracil is a common and naturally occurring pyrimidine nucleobase in which the pyrimidine ring is substituted with two oxo groups at positions 2 and 4. Found in RNA, it base pairs with adenine and replaces thymine during DNA transcription.
How are pyrimidines synthesized?
Pyrimidine is synthesized as a free ring and then a ribose-5-phosphate is added to yield direct nucleotides, whereas, in purine synthesis, the ring is made by attaching atoms on ribose-5-phosphate. The first three enzymes and the fifth and sixth enzymes are part of two multifunctional peptides to increase efficiency.
Why are pyrimidines called bases?
Similarly, the simple-ring structure of cytosine, uracil, and thymine is derived of pyrimidine, so those three bases are called the pyrimidine bases. Each of the base pairs in a typical double-helix DNA comprises a purine and a pyrimidine: either an A paired with a T or a C paired with a G.
What are pyrimidines in DNA?
The pyrimidines found in DNA are Cytosine (C) and Thymine (T). They are capable of complimentary base pairing with the purines Guanine (G) and Aden...
What are the three pyrimidine bases?
Cytosine, thymine, and uracil are the three pyrimidine bases. Cytosine is found in both DNA and RNA, thymine is present only in DNA, and uracil is...
What are the pyrimidine bases and their structures?
The pyrimidine bases are modified pyrimidines which form the nucleotides of DNA and RNA. They are aromatic heterocyclic compounds composed of a six...
What is the pyrimidine ring system?
Pinner's 1885 structure for pyrimidine. The pyrimidine ring system has wide occurrence in nature as substituted and ring fused compounds and derivatives, including the nucleotides cytosine, thymine and uracil, thiamine (vitamin B1) and alloxan. It is also found in many synthetic compounds such as barbiturates and the HIV drug, zidovudine.
When was pyrimidin first used?
Pinner first proposed the name “pyrimidin” in 1885. The parent compound was first prepared by Gabriel and Colman in 1900, by conversion of barbituric acid to 2,4,6-trichloropyrimidine followed by reduction using zinc dust in hot water.
What are the pairs of adenine and guanine?
Thus, in DNA, the purines adenine (A) and guanine (G) pair up with the pyrimidines thymine (T) and cytosine (C), respectively. In RNA, the complement of adenine (A) is uracil (U) instead of thymine (T), so the pairs that form are adenine: uracil and guanine: cytosine .
What is the reaction of urea and amidines to give pyrimidines?
Pyrimidines can be prepared via the Biginelli reaction.
What is RNA made of?
RNA is composed of pyrimidine and purine nucleotides, both of which are necessary for reliable information transfer, and thus natural selection and Darwinian evolution. Becker et al. showed how pyrimidine nucleosides can be synthesized from small molecules and ribose, driven solely by wet-dry cycles.
Why does protonation occur at only one nitrogen?
Protonation and other electrophilic additions will occur at only one nitrogen due to further deactivation by the second nitrogen. The 2-, 4-, and 6- positions on the pyrimidine ring are electron deficient analogous to those in pyridine and nitro- and dinitrobenzene.
Where are uracil and cytosine found?
In March 2015, NASA Ames scientists reported that, for the first time, complex DNA and RNA organic compounds of life, including uracil, cytosine and thymine, have been formed in the laboratory under outer space conditions, using starting chemicals, such as pyrimidine, found in meteorites. Pyrimidine, like polycyclic aromatic hydrocarbons (PAHs), the most carbon-rich chemical found in the universe, may have been formed in red giants or in interstellar dust and gas clouds.
What are the compounds in pyrimidine?
Some well-known pyrimidine compounds include cytosine, thymine, and uracil, present in nucleic acids; thiamine(vitamin B1); and sulfadiazine, sulfamerazine, and sulfamethazine, drugs used in therapy of bacterial and viral diseases. Read More on This Topic.
When were pyrimidines first discovered?
Several pyrimidine compounds were isolated between 1837 and 1864, but their structures were not recognized until 1868. Some well-known pyrimidine compounds include cytosine, thymine, and uracil, present in nucleic acids; thiamine (vitamin B 1 ); and sulfadiazine, sulfamerazine, and sulfamethazine, drugs used in therapy of bacterial and viral diseases.
Which pathway is simpler, pyrimidine or purine?
The biosynthetic pathway for the pyrimidine nucleotides is somewhat simpler than that for the purine nucleotides.
What are the building blocks of DNA?
Purines and pyrimidines are essential building blocks of DNA, RNA, and compounds involved in cellular energy transfer and biosynthetic reactions (e.g., adenosine triphosphate, ATP). Purine and pyrimidine disorders have a wide spectrum of signs and symptoms, including autism, kidney stones, susceptibility to infections,….
What are Pyrimidines?
A pyrimidine is an organic compound known as an aromatic heterocyclic compound and has the molecular formula of C 4 H 4 N 2. Heterocyclic compounds are stable, ring-shaped compounds in which not all atoms in the ring are carbon (Figure 1) .
Pyrimidine Bases
There are three pyrimidine bases found in nucleic acids: thymine (T), cytosine (C), and Uracil (U). These bases are substituted pyrimidines as they have molecular formulas which have been slightly altered from the generic C 4 H 4 N 2 formula (Figure 2).
Pyrimidines in DNA
DNA (deoxyribonucleic acid) contains the pyrimidines cytosine and thymine. Uracil is not present in DNA. In DNA, pyrimidines will form hydrogen bonds with another class of nucleotides, known as purines.
What are pyrimidine rings made of?
Pyrimidine rings are assembled from bicarbonate, aspartate, and Ammonia. The pyrimidine biosynthesis (de novo pyrimidine synthesis pathway) was first observed in mutants of bread mole Neurospora Crassa, which are unable to synthesize pyrimidine, therefore, require both cytosine and Uracil in their growth medium.
What are the three pyrimidine derivatives?
The Synthesis of pyrimidine derivatives are TTP, CTP and UTP.
How is CTP formed?
CTP is formed by the amination of UTP by CTP synthetase. In animals, the amino group is donated by Glutamine whereas in bacteria it is supplied directly in Ammonia. The Synthesis of pyrimidine derivatives are TTP, CTP and UTP.
What is the reaction of OROTATE and PRPP?
Orotate reacts with PRPP to yield Orotidine-5-MonoPhosphate (OMP).
How many nucleotides are in pyrimidines?
In the Pyrimidines, there are three Nucleotide molecules; they are UTP, CTP, and TTP. The De novo pyrimidine synthesis pathway can be explained by the following steps. Synthesis of Carbamoyl Phosphate. Synthesis of Carbamoyl Aspartate. Ring Closure to form dihydroorotate.
What mechanism is used to synthesize UTP?
The synthesis of UTP forms UMP done by phosphate exchange mechanism.
Is pyrimidine a purine?
The pyrimidine synthesis is a similar process than that of Purines ( Purines Synthesis ). In the de novo synthesis of Pyrimidines, the ring is synthesized first and then it is attached to a ribose-phosphate to for a pyrimidine nucleotide. Pyrimidine rings are assembled from bicarbonate, aspartate, and Ammonia.
How many atoms are in pyrimidine?
A pyrimidine is an organic ring consisting of six atoms: 4 carbon atoms and 2 nitrogen atoms. The nitrogen atoms are placed in the 1 and 3 positions around the ring. Atoms or groups attached to this ring distinguish pyrimidines, which include cytosine, thymine, uracil, thiamine (vitamin B1), uric acid, and barbituates.
What are purines made of?
Purines are the most widely occurring heterocyclic molecules that contain nitrogen. They are abundant in meat, fish, beans, peas, and grains. Examples of purines include caffeine, xanthine, hypoxanthine, uric acid, theobromine, ...
What are the end products of purine catabolism?
The end product of purine catabolism is uric acid, while the end products of pyrimidine catabolism are ammonia and carbon dioxide. The body does not make the two molecules in the same location, either. Purines are synthesized primarily in the liver, while a variety of tissues make pyrimidines.
What are the two molecules that form hydrogen bonds?
While purines and pyrimidines include molecules that are active on their own (as in drugs and vitamins), they also form hydrogen bonds between each other to link the two strands of the DNA double helix and to form complementary molecules between DNA and RNA. In DNA, the purine adenine bonds to the pyrimidine thymine and the purine guanine bonds to the pyrimidine cytosine. In RNA, adenine bonds to uracil and guanine still bonds with cytosine. Approximately equal amounts of purines and pyrimidines are required to form either DNA or RNA.
Why do purines have a higher molecular weight?
Obviously, because purines consist of two rings rather than one, they have a higher molecular weight. The ring structure also affects the melting points and solubility of the purified compounds. The human body synthesizes ( anabolism) and breaks down (catabolism) the molecules differently.
Is pyrimidine a benzene?
Pyridine, in turn, is related to benzene ( C 6 H 6 ), except one of the carbon atoms is replaced by a nitrogen atom. Purines and pyrimidines are important molecules in organic chemistry and biochemistry because they are the basis for other molecules (e.g., caffeine, theobromine, theophylline, thiamine) and because they are key components ...
Is pyrimidine an aromatic compound?
Anne Marie Helmenstine, Ph.D. Updated March 27, 2019. Purines and pyrimidines are two types of aromatic heterocyclic organic compounds. In other words, they are ring structures (aromatic) that contain nitrogen as well as carbon in the rings (heterocyclic).
What are the derivatives of pyrimidone?
Derivatives of pyrimidone are the basis of many other biological molecules, including: Antiulcer drugs including temelastine, icotidine, donetidine, and lupitidine. This article about a heterocyclic compound is a stub. You can help Wikipedia by expanding it.
What is the name of the compound that is a heterocyclic compound?
Chemical compound. Pyrimidone is the name given to either of two heterocyclic compounds with the formula C 4 H 4 N 2 O: 2-pyrimidone and 4-pyrimidone. The compounds can also be called 2-hydroxypyrimidine or 4-hydroxypyrimidine respectively, based on a substituted pyrimidine, or 1,3- diazine, ring.
What is the second step in pyrimidine synthesis?
The second step in pyrimidine synthesis is the formation of carbamoylaspartate, catalyzed by aspartate transcarbamoylase. The pyrimidine ring is then closed by dihydroorotase. The resulting dihydroorotate is oxidized to produce orotic acid (orotate) as shown in Figure 22.21. The enzyme that produces orotate, dihydroorotate dehydrogenase, is a flavoprotein associated with the inner mitochondrial membrane. All other enzymes in pyrimidine biosynthesis are cytosolic. [Note: The first three enzymic activities in this pathway (CPS II, aspartate transcarbamoylase, and dihydroorotase) are actually three different catalytic domains of a single polypeptide known as CAD from the first letter in the name of each domain. This is an example of a multfunctional or multicatalytic polypeptide that facilitates the ordered synthesis of an important compound. Synthesis of the purine nucleotide IMP also involves multifunctional proteins.]
What is the regulated step of the pyrimidine pathway?
The regulated step of this pathway in mammalian cells is the synthesis of carbamoyl phosphate from glutamine and CO2, catalyzed by carbamoyl phosphate synthetase (CPS) II. CPS II is inhibited by uridine triphosphate (the end product of this pathway, which can be converted into the other pyrimidine nucleotides), and is activated by PRPP. [Note: Carbamoyl phosphate, synthesized by CPS I, is also a precursor of urea. Defects in ornithine transcarbamylase of the urea cycle promote pyrimidine synthesis due to increased availability of carbamoyl phosphate. A comparison of the two enzymes is presented in Figure 22.20.]
How is cytidine triphosphate produced?
Cytidine triphosphate (CTP) is produced by amination of UTP by CTP synthetase (Figure 22.22) , with glutamine providing the nitrogen. [Note: Some CTP is dephosphorylated to cytidine diphosphate (CDP), which is a substrate for ribonucleotide reductase. The dCDP product can be phosphorylated to dCTP for DNA synthesis or dephosphoprylated to dCMP that is deaminated to dUMP.]
Is pyrimidine a phosphorylated nucleotide?
Unlike the purine ring, which is not cleaved in humans, the pyrimidine ring is opened and degraded to highly soluble products, β-alanine (from the degradation of CMP and UMP) and β-aminoisobutyrate (from TMP degradation), with the production of NH3 and CO2. Pyrimidine bases can be salvaged to nucleosides, which are phosphorylated to nucleotides. However, their high solubility makes pyrimidine salvage less significant clinically than purine salvage. [Note: The salvage of pyrimidine nucleosides is the basis for using uridine in the treatment of hereditary orotic aciduria.]
What is Pyridium?
Pyridium ( phenazopyridine) is a pain reliever that affects the lower part of your urinary tract (bladder and urethra).
How should I take Pyridium?
Use Pyridium exactly as directed on the label, or as prescribed by your doctor. Do not use in larger or smaller amounts or for longer than recommended.
What are the side effects of pyridium?
blue or purple appearance of your skin. Common Pyridium side effects may include: headache; dizziness; or. upset stomach. This is not a complete list of side effects and others may occur. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. Pyridium side effects (more detail)
Does pyridium cause nausea?
To avoid stomach upset, take this medicine with food. Pyridium will most likely darken the color of your urine to an orange or red color. This is a normal effect and is not cause for alarm unless you have other symptoms such as pale or yellowed skin, fever, stomach pain, nausea, and vomiting.
Is pyridium safe for babies?
Before taking this medicine. To make sure Pyridium is safe for you, tell your doctor if you have ever had: liver disease; diabetes; or. a genetic enzyme deficiency called glucose-6-phosphate dehydrogenase (G6PD) deficiency. Pyridium is not expected to harm an unborn baby.
Can pyridium cause hives?
Pyridium side effects. Get emergency medical help if you have any of these signs of an allergic reaction to Pyridium: hives; difficult breathing; swelling of your face, lips, tongue, or throat. Stop using Pyridium and call your doctor at once if you have: blue or purple appearance of your skin.
Can you take pyridium if you are allergic to phenazopyridine?
Do not take Pyridium if you are allergic to phenazopyridine, or if you have kidney disease. Pyridium will treat the symptoms of a urinary tract infection, but this medication does not treat the actual infection. Take any antibiotic that your doctor prescribes to treat your infection.
Overview
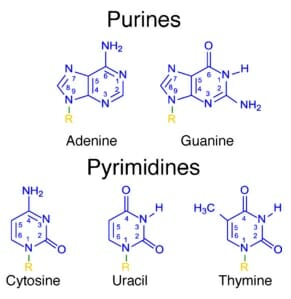
Pyrimidine is an aromatic heterocyclic organic compound similar to pyridine. One of the three diazines (six-membered heterocyclics with two nitrogen atoms in the ring), it has the nitrogen atoms at positions 1 and 3 in the ring. The other diazines are pyrazine (nitrogen atoms at the 1 and 4 positions) and pyridazine (nitrogen atoms at the 1 and 2 positions). In nucleic acids, three types of nucleobases are pyrimidine derivatives: cytosine (C), thymine (T), and uracil (U).
Occurrence and history
The pyrimidine ring system has wide occurrence in nature as substituted and ring fused compounds and derivatives, including the nucleotides cytosine, thymine and uracil, thiamine (vitamin B1) and alloxan. It is also found in many synthetic compounds such as barbiturates and the HIV drug, zidovudine. Although pyrimidine derivatives such as alloxan were known in the early 19th century, a laboratory synthesis of a pyrimidine was not carried out until 1879, when Grimaux rep…
Nomenclature
The nomenclature of pyrimidines is straightforward. However, like other heterocyclics, tautomeric hydroxyl groups yield complications since they exist primarily in the cyclic amide form. For example, 2-hydroxypyrimidine is more properly named 2-pyrimidone. A partial list of trivial names of various pyrimidines exists.
Physical properties
Physical properties are shown in the data box. A more extensive discussion, including spectra, can be found in Brown et al.
Chemical properties
Per the classification by Albert six-membered heterocycles can be described as π-deficient. Substitution by electronegative groups or additional nitrogen atoms in the ring significantly increase the π-deficiency. These effects also decrease the basicity.
Like pyridines, in pyrimidines the π-electron density is decreased to an even greater extent. Therefore, electrophilic aromatic substitution is more difficult while nucleophilic aromatic substit…
Synthesis
As is often the case with parent heterocyclic ring systems, the synthesis of pyrimidine is not that common and is usually performed by removing functional groups from derivatives. Primary syntheses in quantity involving formamide have been reported.
As a class, pyrimidines are typically synthesized by the principal synthesis invo…
Reactions
Because of the decreased basicity compared to pyridine, electrophilic substitution of pyrimidine is less facile. Protonation or alkylation typically takes place at only one of the ring nitrogen atoms. Mono-N-oxidation occurs by reaction with peracids.
Electrophilic C-substitution of pyrimidine occurs at the 5-position, the least electron-deficient. Nitration, nitrosation, azo coupling, halogenation, sulfonation, formylation, hydroxymethylation, an…
Derivatives
Three nucleobases found in nucleic acids, cytosine (C), thymine (T), and uracil (U), are pyrimidine derivatives:
Cytosine (C) Thymine (T) Uracil (U)
In DNA and RNA, these bases form hydrogen bonds with their complementary purines. Thus, in DNA, the purines adenine (A) and guanine (G) pair up with the p…