
How can I calculate gas pressure?
pressure1 × volume1 = pressure2 × volume2 Note that volume is measured in metres cubed (m3) and temperature in kelvin (K). It means that for a gas at a constant temperature, pressure × volume is...
How do you set gas pressure on a gas valve?
Set the high pressure to 3.5 inches of water column (WC) for natural gas.
- For a propane furnace, the valve has to be set to 11 inches of WC or a similar number.
- Keep an eye on the manometer, since its display will change as you adjust the valve.
- Tune the gas pressure gradually. For safety, tune it to the pressure rating recommended by the manufacturer.
What is the equation for gas pressure?
The mathematical expression for the pressure law: that is, the ratio, P/T = constant, when the volume is kept constant. The equation for Pressure law is: The relationship between the pressure and the absolute temperature of a gas under constant volume can also be expressed by the graphs in Figure. People also ask.
What is ideal gas pressure?
Ideal Gas Law Ideal Gas Law PV = nRT The pressure of a gas times its volume equals the number of moles of the gas times a constant (R) times the temperature of the gas. The ideal gas law is the final and most useful expression of the gas laws because it ties the amount of a gas (moles) to its pressure, volume and temperature.
What is the unit of pressure?
How many quantities are needed for a complete physical description of a sample of a gas?
How does the atmosphere exert pressure?
How does pressure affect the size of the area?
What is the force of air in a balloon?
How much does each square meter of Earth's surface weigh?
Does it take a taller column of a less dense liquid to achieve the same pressure?
See 4 more
About this website
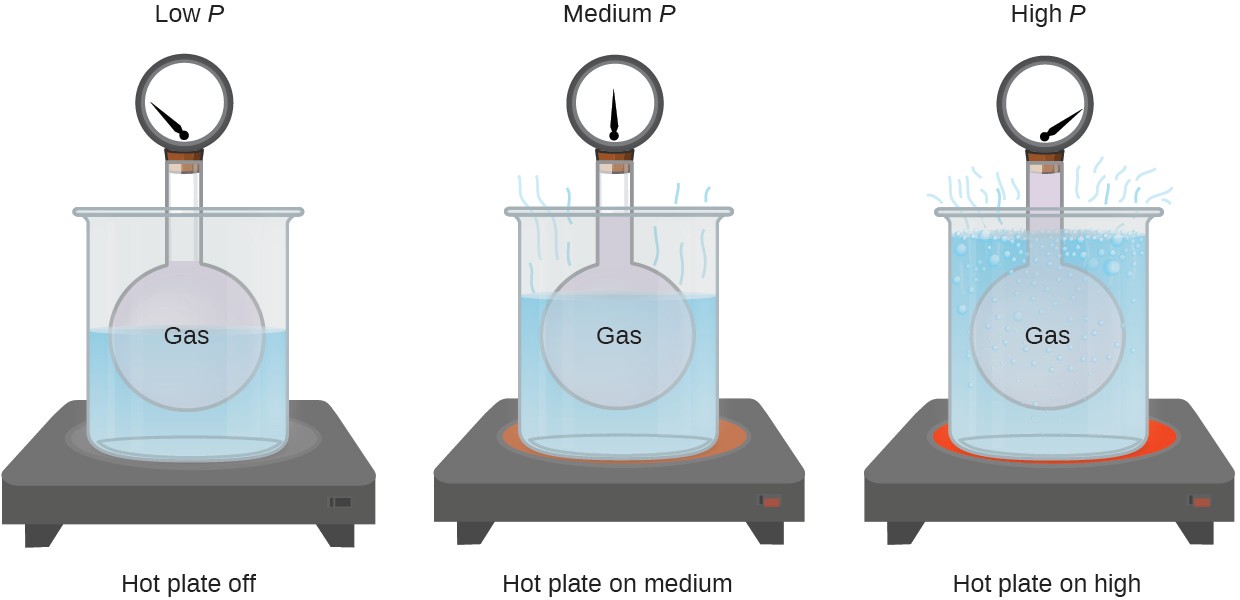
What describes the pressure of a gas?
The sum of the forces of all the molecules striking the wall divided by the area of the wall is defined to be the pressure. The pressure of a gas is then a measure of the average linear momentum of the moving molecules of a gas.
What is used for measuring gas pressure?
manometerA manometer is an apparatus used to measure the pressure of a sample of a gas.
What is the measurement of gas?
cubic feetGas is sometimes measured in cubic feet at a temperature of 60 degrees Fahrenheit and an atmospheric pressure of 14.7 pounds per square inch. Gas production from wells is discussed in terms of thousands or millions of cubic feet (Mcf and MMcf). Resources and reserves are calculated in trillions of cubic feet (Tcf).
How is natural gas pressure measured?
1:454:29Checking Natural Gas Manifold Pressure - YouTubeYouTubeStart of suggested clipEnd of suggested clipAnd we'll make sure that we're in inches. Of water column. So before we go ahead and check theMoreAnd we'll make sure that we're in inches. Of water column. So before we go ahead and check the manifold pressure we want to come over to the nameplate.
What is the unit of measurement for natural gas pressure?
Gas Pressure is measured in a variety of units. Standard Temperature and pressure call for one atmosphere (1 atm) at 0oC which is 273 K. In physics, the measure is completed in units called Pascals where 1 atm of gas is equivalent to 101,325 Pa or 101.3 kPa (kiloPascals).
What is pressure measured in?
Pascal (Pa)The standard SI unit for pressure measurement is the Pascal (Pa) which is equivalent to one Newton per square meter (N/m2) or the KiloPascal (kPa) where 1 kPa = 1000 Pa. In the English system, pressure is usually expressed in pounds per square inch (psi).
What is the unit of pressure?
The SI unit of pressure is pascal (represented as Pa) which is equal to one newton per square metre (N/m2 or kg m-1s-2).
What does the pressure of gas measure quizlet?
Gas pressure is the force of its outward push divided by the area of the walls of its container. As the moving gas molecules collide with the walls of their container, they push on the container walls. You just studied 10 terms!
What is a manometer used for?
The manometer is so often used to measure pressure that the difference in column heights is also a common unit. This is expressed in inches or centimeters of water or mercury at a specific temperature, which can be changed to standard units of pressure with a conversion table.
What instrument measures a low gas pressure?
pressure gauge, instrument for measuring the condition of a fluid (liquid or gas) that is specified by the force that the fluid would exert, when at rest, on a unit area, such as pounds per square inch or newtons per square centimetre.
What is barometer and manometer?
Barometers and manometers are devices used to measure pressure. • The simplest forms of these devices use the height of a column of liquid with a known density to indicate the pressure. • The height of the column is a consequence of the static force due to gravity acting on the liquid.
What tool measures gas velocity?
Velocity Turbine flow meters measure volumetric flow based on fluid flowing past a free-spinning rotor, each revolution corresponding to a specific volume of gas or liquid. The meters have high turndown and accuracy.
Phet Gas Law Simulation Answers.pdf - Phet Gas Law...
Phet Gas Law Simulation Answers Yeah, reviewing a ebook phet gas law simulation answers could mount up your near connections listings. This is just one of the solutions for you to be successful. As understood, skill does not recommend that you have astounding points. Comprehending as well as accord even more than supplementary will have the funds for each success. next-door to, the publication ...
Gas Properties Homework Activity Answers - | JILA
Page 2 Pause the simulation. Under “Measurement Tools” choose “ruler”. The ruler is in units of nanometers. Click and drag the ruler to measure the diameter of a heavy molecule.
2011 Gas properties activity answers - | JILA
2 Assumptions to the ideal gas law 1. Molecules are volumeless 2. No attractive forces between molecules No longer true: molecules are colliding and have a finite volume
Gas Law Simulator - University of Texas at Austin
Gas Law Simulator Multiple Panels - pressure, volume, temperature, kinetic energy, and RMS velocity
How much CH4 is in a gas sample?
A gas sample contains 4.0 g of CH4 and 2.0 g of He. What is the volume of the sample at STP?
How much KI is in 100 g of H2O?
The solubility of KI is 50 g in 100 g of H2O at 20 °C. If 110 grams of KI are added to 200 grams of H2O
What is the unit of pressure?
The units of pressure are derived from the units used to measure force and area. The SI unit for pressure, derived from the SI units for force (newtons) and area (square meters), is the newton per square meter ( N / m 2 ), which is called the Pascal (Pa), after the French mathematician Blaise Pascal (1623–1662):
How many quantities are needed for a complete physical description of a sample of a gas?
At the macroscopic level, a complete physical description of a sample of a gas requires four quantities:
How does the atmosphere exert pressure?
Just as we exert pressure on a surface because of gravity, so does our atmosphere. We live at the bottom of an ocean of gases that becomes progressively less dense with increasing altitude. Approximately 99% of the mass of the atmosphere lies within 30 km of Earth’s surface (Figure 5.2. 1 ). Every point on Earth’s surface experiences a net pressure called barometric pressure. The pressure exerted by the atmosphere is considerable: a 1 m 2 column, measured from sea level to the top of the atmosphere, has a mass of about 10,000 kg, which gives a pressure of about 101 kPa:
How does pressure affect the size of the area?
Pressure is dependent on both the force exerted and the size of the area to which the force is applied. We know from Equation 5.2.1 that applying the same force to a smaller area produces a higher pressure. When we use a hose to wash a car, for example, we can increase the pressure of the water by reducing the size of the opening of the hose with a thumb.
What is the force of air in a balloon?
The air in a balloon, for example, exerts a force against the interior surface of the balloon, and a liquid injected into a mold exerts a force against the interior surface of the mold, just as a chair exerts a force against the floor because of its mass and the effects of gravity. If the air in a balloon is heated, ...
How much does each square meter of Earth's surface weigh?
Figure 5.2. 1: Barometric Pressure. Each square meter of Earth’s surface supports a column of air that is more than 200 km high and weighs about 10,000 kg at Earth’s surface.
Does it take a taller column of a less dense liquid to achieve the same pressure?
The answer makes sense: it takes a taller column of a less dense liquid to achieve the same pressure.
